Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DUOX13
|
|||
| Drug Name |
ENB003
|
|||
| Synonyms |
AAY8R26VDX; Vodudeutentan sodium; BQ-788, di(methyl-d)-; UNII-AAY8R26VDX; ENB003; ENB-003; 2364572-10-9; D-Norleucine, N-((cis-2,6-di(methyl-d)-1-piperidinyl)carbonyl)-4-methyl-L-leucyl-1-(methoxycarbonyl)-D-tryptophyl-, sodium salt; Enb 003; CHEMBL5095234; ENB 003 [WHO-DD]; BQ 788-d2
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 1/2 | [1] | |
| Company |
ENB Therapeutics New York, NY
|
|||
| Structure |
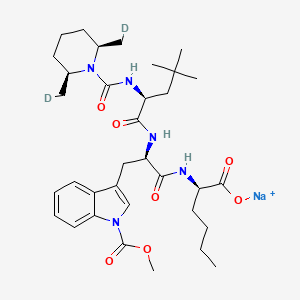 |
Download2D MOL
|
||
| Formula |
C34H50N5NaO7
|
|||
| Canonical SMILES |
CCCCC(C(=O)[O-])NC(=O)C(CC1=CN(C2=CC=CC=C21)C(=O)OC)NC(=O)C(CC(C)(C)C)NC(=O)N3C(CCCC3C)C.[Na+]
|
|||
| InChI |
InChI=1S/C34H51N5O7.Na/c1-8-9-16-25(31(42)43)35-29(40)26(18-23-20-38(33(45)46-7)28-17-11-10-15-24(23)28)36-30(41)27(19-34(4,5)6)37-32(44)39-21(2)13-12-14-22(39)3;/h10-11,15,17,20-22,25-27H,8-9,12-14,16,18-19H2,1-7H3,(H,35,40)(H,36,41)(H,37,44)(H,42,43);/q;+1/p-1/t21-,22+,25-,26-,27+;/m1./s1/i2D,3D;
|
|||
| InChIKey |
QCVIFBRTTLMEOV-ZOHSNMQTSA-M
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Endothelin B receptor (EDNRB) | Target Info | Antagonist | [1] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Melanogenesis | ||||
| Pathways in cancer | ||||
| Panther Pathway | Endothelin signaling pathway | |||
| Pathway Interaction Database | Endothelins | |||
| Arf6 trafficking events | ||||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Peptide GPCRs | ||||
| Endothelin Pathways | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04205227) A Phase 1/2A Trial of ENB 003 in Combination With Pembrolizumab in Subjects With Advanced Solid Tumors. U.S.National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

