Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DS6V8H
|
|||
| Drug Name |
SAR 444727
|
|||
| Synonyms |
atuzabrutinib; Atuzabrutinib [INN]; YZ68ZB8LWA; UNII-YZ68ZB8LWA; PRN473; CHEMBL4114766; PRN-473; SAR444727; SAR-444727; 1581714-49-9; (alphaE,3R)-3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1hpyrazolo(3,4-d)pyrimidin-1-yl)-alpha-(2,2-dimethylpropylidene)-beta-oxo-1-piperidinepropanenitrile; 1-Piperidinepropanenitrile, 3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidin-1-yl)-alpha-(2,2-dimethylpropylidene)-beta-oxo-, (alphaE,3R)-; 1-Piperidinepropanenitrile, 3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-alpha-(2,2-dimethylpropylidene)-beta-oxo-, (alphaE,3R)-; ATUZABRUTINIB [USAN]; SCHEMBL15515897; SCHEMBL15516108; GTPL11666; BDBM197260; BDBM50589191; AKOS040756908; compound 11 [PMID: 35302767]; HY-132808; CS-0204055; US9090621, 125A; (2E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile; (alphaE,3R)-3-[4-Amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-alpha-(2,2-dimethylpropylidene)-beta-oxo-1-piperidinepropanenitrile; (E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Atopic dermatitis [ICD-11: EA80; ICD-10: L20] | Phase 2 | [1] | |
| Structure |
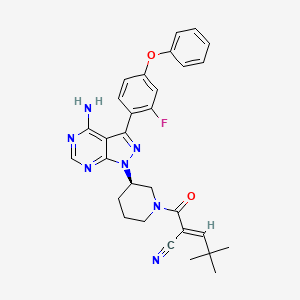 |
Download2D MOL |
||
| Formula |
C30H30FN7O2
|
|||
| Canonical SMILES |
CC(C)(C)C=C(C#N)C(=O)N1CCCC(C1)N2C3=NC=NC(=C3C(=N2)C4=C(C=C(C=C4)OC5=CC=CC=C5)F)N
|
|||
| InChI |
InChI=1S/C30H30FN7O2/c1-30(2,3)15-19(16-32)29(39)37-13-7-8-20(17-37)38-28-25(27(33)34-18-35-28)26(36-38)23-12-11-22(14-24(23)31)40-21-9-5-4-6-10-21/h4-6,9-12,14-15,18,20H,7-8,13,17H2,1-3H3,(H2,33,34,35)/b19-15+/t20-/m1/s1
|
|||
| InChIKey |
KZMQPYCXSAGLTB-ZWUNQBBJSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04992546) A Randomized, Intra-patient, Double-blind, Placebo-controlled Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Topically Administered PRN473 (SAR444727) in Patients With Mild to Moderate Atopic Dermatitis. U.S.National Institutes of Health. | |||
| REF 2 | Preclinical Mechanisms of Topical PRN473, a Bruton Tyrosine Kinase Inhibitor, in Immune-Mediated Skin Disease Models. Immunohorizons. 2021 Jul 29;5(7):581-589. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

