Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DQ84IT
|
|||
| Drug Name |
ICLUSIG
|
|||
| Synonyms |
Ponatinib hydrochloride; 1114544-31-8; Ponatinib HCl; Iclusig; AP 24534 hydrochloride; AP24534 HCL; Ponatinib Mono-hydrochloride; UNII-96R6PU3D8J; 96R6PU3D8J; Ponatinib hydrochloride [USAN]; AP-24534 HCl; Ponatinib (hydrochloride); AP-24534 hydrochloride; CHEBI:191940; DTXSID50149655; 1114544-31-8 (HCl); 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide hydrochloride; 3-(2-(Imidazo(1,2-b)pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1- yl)methyl)-3-(trifluoromethyl)phenyl)benzamide monohydrochloride; Benzamide, 3-(2-imidazo(1,2-b)pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methyl-1- piperazinyl)methyl)-3-(trifluoromethyl)phenyl)-, hydrochloride (1:1); C29H28ClF3N6O; 3-((imidazo(1,2-b)pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide--hydrogen chloride; 3-(2-(Imidazo[1,2-b]pyridazin-3-yl)ethynyl)-4-methyl-n-(4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl)benzamide hydrochloride; 3-(2-{imidazo[1,2-b]pyridazin-3-yl}ethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide hydrochloride; 3-(imidazo(1,2-b)pyridazin-3-ylethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide hydrochloride; 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide hydrochloride; 3-[(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide--hydrogen chloride; 4-((4-(3-((imidazo(1,2-b)pyridazin-3-yl)ethynyl)-4-methylbenzamido)-2-(trifluoromethyl)phenyl)methyl)-1-methylpiperazin-1-ium chloride; 4-(4-(3-(imidazo(1,2-b)pyridazin-3-ylethynyl)-4-methylbenzamido)-2-(trifluoromethyl)benzyl)-1-methylpiperazin-1-ium chloride; 4-{[4-{3-[(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methylbenzamido}-2-(trifluoromethyl)phenyl]methyl}-1-methylpiperazin-1-ium chloride; 4-{4-[3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylbenzamido]-2-(trifluoromethyl)benzyl}-1-methylpiperazin-1-ium chloride; Iclusig (TN); Ponatinib (HCl salt); Ponatinib (2 x HCl Salt); AMY367; AP 24534 HCl; CHEMBL2105708; DTXCID0072146; SCHEMBL15798559; 943319-70-8 free base; BCP10906; Ponatinib hydrochloride (JAN/USAN); PONATINIB HYDROCHLORIDE [MI]; EX-A067-1; PONATINIB HYDROCHLORIDE [JAN]; AKOS030621539; PONATINIB HYDROCHLORIDE [WHO-DD]; AC-32047; AS-16772; HY-108766; CS-0031009; PONATINIB HYDROCHLORIDE [ORANGE BOOK]; D09951; Q27271925; 3-(2-(Imidazo(1,2-b)pyridazin-3-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide monohydrochloride; 3-(2-imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]benzamide;hydrochloride; Benzamide, 3-(2-Imidazo(1,2-B)Pyridazin-3-Ylethynyl)-4-Methyl-N-(4-((4-Methyl-1-Piperazinyl)Methyl)-3-(Trifluoromethyl)Phenyl)-, Hydrochloride (1:1)
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Acute lymphoblastic leukaemia [ICD-11: 2A85; ICD-10: C91.0] | Phase 3 | [1] | |
| Company |
Takeda
|
|||
| Structure |
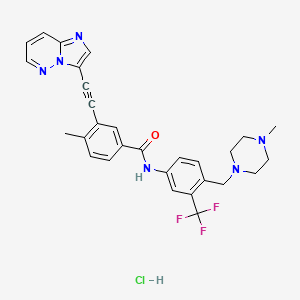 |
Download2D MOL
|
||
| Formula |
C29H28ClF3N6O
|
|||
| Canonical SMILES |
CC1=C(C=C(C=C1)C(=O)NC2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)C#CC4=CN=C5N4N=CC=C5.Cl
|
|||
| InChI |
InChI=1S/C29H27F3N6O.ClH/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37;/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39);1H
|
|||
| InChIKey |
BWTNNZPNKQIADY-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fusion protein Bcr-Abl (Bcr-Abl) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03589326) A Phase 3, Randomized, Open-label, Multicenter Study Comparing Ponatinib Versus Imatinib, Administered in Combination With Reduced-Intensity Chemotherapy, in Patients With Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia (Ph+ ALL). U.S.National Institutes of Health. | |||
| REF 2 | Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018 Jul 26;132(4):393-404. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

