Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DQ62LM
|
|||
| Drug Name |
AZD8154
|
|||
| Synonyms |
AZD8154; AZD-8154; 2215022-45-8; J5M3TCK9Y9; UNII-J5M3TCK9Y9; CHEMBL4558527; AZD-8154 [WHO-DD]; 1H-Isoindol-1-one, 2-((1S)-1-cyclopropylethyl)-2,3-dihydro-5-(4-methyl-2-((6-(2-oxo-1-pyrrolidinyl)-2-pyridinyl)amino)-5-thiazolyl)-7-(methylsulfonyl)-; Azd 8154; SCHEMBL22204109; GTPL11204; AZD 8154 [WHO-DD]; EX-A5133; BDBM50512861; AKOS040757410; Example 1 [WO2018055040A1]; MS-30088; HY-115870; CS-0371158; 2-[(1S)-1-cyclopropylethyl]-5-[4-methyl-2-[[6-(2-oxopyrrolidin-1-yl)pyridin-2-yl]amino]-1,3-thiazol-5-yl]-7-methylsulfonyl-3H-isoindol-1-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Phase 2 | [1] | |
| Company |
AstraZeneca Wilmington, DE
|
|||
| Structure |
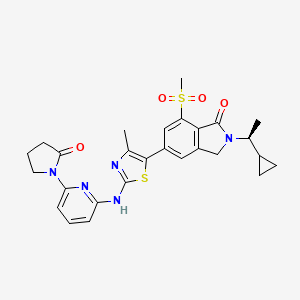 |
Download2D MOL |
||
| Formula |
C27H29N5O4S2
|
|||
| Canonical SMILES |
CC1=C(SC(=N1)NC2=NC(=CC=C2)N3CCCC3=O)C4=CC5=C(C(=C4)S(=O)(=O)C)C(=O)N(C5)C(C)C6CC6
|
|||
| InChI |
InChI=1S/C27H29N5O4S2/c1-15-25(37-27(28-15)30-21-6-4-7-22(29-21)31-11-5-8-23(31)33)18-12-19-14-32(16(2)17-9-10-17)26(34)24(19)20(13-18)38(3,35)36/h4,6-7,12-13,16-17H,5,8-11,14H2,1-3H3,(H,28,29,30)/t16-/m0/s1
|
|||
| InChIKey |
XOMFDZJQLSPGGV-INIZCTEOSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04187508) A Phase IIa, Double-Blind, Randomized, Parallel Group, Placebo-Controlled Multi-Centre Study to Evaluate the Effect of AZD8154 Administered Via Nebulizer Once Daily on Allergen-Induced Inflammation in Subjects With Mild Allergic Asthma Challenged With an Inhaled Allergen. U.S.National Institutes of Health. | |||
| REF 2 | Discovery of AZD8154, a Dual PI3Kgamma-delta Inhibitor for the Treatment of Asthma. J Med Chem. 2021 Jun 24;64(12):8053-8075. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

