Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DP6AO8
|
|||
| Drug Name |
AZD9977
|
|||
| Synonyms |
AZD9977; AZD-9977; 1850385-64-6; BALCINRENONE; UNII-6C9UKZ0CYE; 6C9UKZ0CYE; Azd 9977; 2-[(3S)-7-Fluoro-4-(3-oxo-4H-1,4-benzoxazine-6-carbonyl)-2,3-dihydro-1,4-benzoxazin-3-yl]-N-methylacetamide; 2H-1,4-Benzoxazine-3-acetamide, 4-((3,4-dihydro-3-oxo-2H-1,4-benzoxazin-6-yl)carbonyl)-7-fluoro-3,4-dihydro-N-methyl-, (3S)-; 2-[(3~{S})-7-fluoranyl-4-[(3-oxidanylidene-4~{H}-1,4-benzoxazin-6-yl)carbonyl]-2,3-dihydro-1,4-benzoxazin-3-yl]-~{N}-methyl-ethanamide; 2-((3S)-7-FLUORO-4-((3-OXO-3,4-DIHYDRO-2H-1,4-BENZOXAZIN-6-YL)CARBONYL)-3,4-DIHYDRO-2H-1,4-BENZOXAZIN-3-YL)-N-METHYLACETAMIDE; 2-{(3S)-7-Fluoro-4-[(3-oxo-3,4-dihydro-2H-1,4-benzoxazin-6-yl)carbonyl]-3,4-dihydro-2H-1,4-benzoxazin-3-yl}-N-methylacetamide; BALCINRENONE [INN]; CHEMBL3916929; SCHEMBL17363859; GTPL11281; AZD 9977 [WHO-DD]; MBKYLPOPYYLTNW-ZDUSSCGKSA-N; BDBM238159; AKOS040759623; DB15418; US9394291, 4a; AC-36749; MS-26766; HY-120274; CS-0077469; F82308; (S)-1 [PMID: 30596500]; ECV
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Heart failure [ICD-11: BD10-BD13] | Phase 2 | [1] | |
| Company |
AstraZeneca Wilmington, DE
|
|||
| Structure |
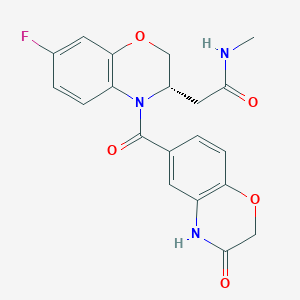 |
Download2D MOL |
||
| Formula |
C20H18FN3O5
|
|||
| Canonical SMILES |
CNC(=O)CC1COC2=C(N1C(=O)C3=CC4=C(C=C3)OCC(=O)N4)C=CC(=C2)F
|
|||
| InChI |
InChI=1S/C20H18FN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26)/t13-/m0/s1
|
|||
| InChIKey |
MBKYLPOPYYLTNW-ZDUSSCGKSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Mineralocorticoid receptor (MR) | Target Info | Modulator | [2] |
| KEGG Pathway | Aldosterone-regulated sodium reabsorption | |||
| Pathwhiz Pathway | Kidney Function | |||
| WikiPathways | ACE Inhibitor Pathway | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04595370) A Phase 2b, Randomised, Double-Blind, Active Controlled, Multi Centre Study to Evaluate the Efficacy, Safety and Tolerability of Oral AZD9977 and Dapagliflozin Treatment in Patients With Heart Failure and Chronic Kidney Disease. U.S.National Institutes of Health. | |||
| REF 2 | The selective mineralocorticoid receptor modulator AZD9977 reveals differences in mineralocorticoid effects of aldosterone and fludrocortisone. J Renin Angiotensin Aldosterone Syst. 2019 Jan-Mar;20(1):1470320319827449. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

