Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DO57QB
|
|||
| Drug Name |
ETX0282
|
|||
| Synonyms |
ETX0282; GTPL10781; ETX-0282; isopropyl (R)-2-(((1R,2R,5R)-2-carbamoyl-4-methyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-3-en-6-yl)oxy)-2-fluoroacetate
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Urinary tract infection [ICD-11: GC08; ICD-10: N39, N39.0] | Phase 1 | [1] | |
| Company |
Entasis Therapeutics Waltham, MA
|
|||
| Structure |
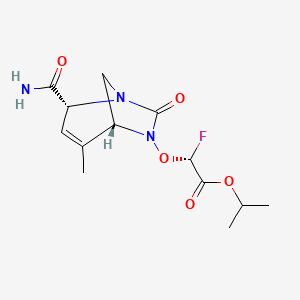 |
Download2D MOL |
||
| Formula |
C13H18FN3O5
|
|||
| Canonical SMILES |
CC1=CC(N2CC1N(C2=O)OC(C(=O)OC(C)C)F)C(=O)N
|
|||
| InChI |
InChI=1S/C13H18FN3O5/c1-6(2)21-12(19)10(14)22-17-9-5-16(13(17)20)8(11(15)18)4-7(9)3/h4,6,8-10H,5H2,1-3H3,(H2,15,18)/t8-,9+,10+/m1/s1
|
|||
| InChIKey |
OMNVFPBGXYKTDB-UTLUCORTSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Staphylococcus Beta-lactamase (Stap-coc blaZ) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03491748) A Phase 1, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Oral ETX0282 Administered in Healthy Subjects. U.S.National Institutes of Health. | |||
| REF 2 | Interactions of the Diazabicyclooctane Serine beta-Lactamase Inhibitor ETX1317 with Target Enzymes. ACS Infect Dis. 2021 Jan 8;7(1):114-122. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

