Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DM61KY
|
|||
| Drug Name |
PF-07081532
|
|||
| Synonyms |
Lotiglipron; PF-07081532; 2401892-75-7; LOTIGLIPRON [USAN]; YI10W1K93A; SCHEMBL22009794; BDBM450633; EX-A7734; US10676465, Example 10; HY-153865; CS-0865718; 1H-Benzimidazole-6-carboxylic acid, 2-[[4-[(2S)-2-(5-chloro-2-pyridinyl)-2-methyl-1,3-benzodioxol-4-yl]-1-piperidinyl]methyl]-1-[(2S)-2-oxetanylmethyl]-; 2-({4-[(S)-2-(5-Chloropyridin-2-yl)-2-methylbenzo[1,3]dioxol-4-yl]piperidin-1-yl}methyl)-1-{[(S)-oxetan-2-yl]methyl}-1H-benzimidazole-6-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Obesity [ICD-11: 5B81; ICD-10: E66] | Phase 2 | [1] | |
| Type 2 diabetes [ICD-11: 5A11; ICD-10: E08-E13] | Phase 2 | [1] | ||
| Company |
Pfzer
|
|||
| Structure |
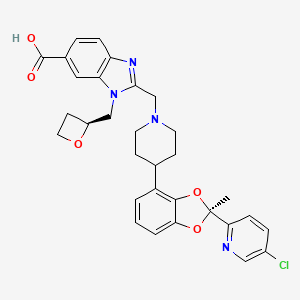 |
Download2D MOL |
||
| Formula |
C31H31ClN4O5
|
|||
| Canonical SMILES |
CC1(OC2=CC=CC(=C2O1)C3CCN(CC3)CC4=NC5=C(N4CC6CCO6)C=C(C=C5)C(=O)O)C7=NC=C(C=C7)Cl
|
|||
| InChI |
InChI=1S/C31H31ClN4O5/c1-31(27-8-6-21(32)16-33-27)40-26-4-2-3-23(29(26)41-31)19-9-12-35(13-10-19)18-28-34-24-7-5-20(30(37)38)15-25(24)36(28)17-22-11-14-39-22/h2-8,15-16,19,22H,9-14,17-18H2,1H3,(H,37,38)/t22-,31-/m0/s1
|
|||
| InChIKey |
SVPYZAJTWFQTSM-UGDMGKLASA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucagon-like peptide 1 receptor (GLP1R) | Target Info | Agonist | [1] |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Insulin secretion | ||||
| Reactome | Glucagon-like Peptide-1 (GLP1) regulates insulin secretion | |||
| G alpha (s) signalling events | ||||
| Glucagon-type ligand receptors | ||||
| WikiPathways | GPCRs, Class B Secretin-like | |||
| Integration of energy metabolism | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05579977) A PHASE 2, RANDOMIZED, DOUBLE-BLIND, PLACEBO CONTROLLED, DOSE RANGING, DOSE FINDING, PARALLEL GROUP STUDY TO ASSESS EFFICACY AND SAFETY OF PF-07081532, AND OPEN LABEL ORAL SEMAGLUTIDE, IN ADULTS WITH TYPE 2 DIABETES MELLITUS (T2DM) INADEQUATELY CONTROLLED ON METFORMIN, AND SEPARATELY PF-07081532 COMPARED TO MATCHING PLACEBO IN ADULTS WITH OBESITY BUT WITHOUT T2DM. U.S.National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

