Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DKZ07O
|
|||
| Drug Name |
Elinzanetant
|
|||
| Synonyms |
Elinzanetant; 929046-33-3; NT-814; UNII-NZW2BOW35N; BAY3427080; Elinzanetant [INN]; Elinzanetant [USAN]; NZW2BOW35N; BAY-3427080; N-[6-[(7S,9aS)-7-(hydroxymethyl)-3,4,6,7,9,9a-hexahydro-1H-pyrazino[2,1-c][1,4]oxazin-8-yl]-4-(4-fluoro-2-methylphenyl)pyridin-3-yl]-2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethylpropanamide; 2-(3,5-BIS(TRIFLUOROMETHYL)PHENYL)-N-(4-(4-FLUORO-2-METHYLPHENYL)-6-((7S,9AS)-7-(HYDROXYMETHYL)HEXAHYDROPYRAZINO(2,1-C)(1,4)OXAZIN-8(1H)-YL)-3-PYRIDINYL)-N,2-DIMETHYLPROPANAMIDE; 2-[3,5-Bis(trifluoromethyl)phenyl]-N-{4-(4-fluoro-2-methylphenyl)-6-[(7S,9aS)-7-(hydroxymethyl)hexahydropyrazino[2,1-c][1,4]oxazin-8(1H)-yl]-3-pyridinyl}-N,2-dimethylpropanamide; Elinzanetant [USAN:INN]; ELINZANETANT [WHO-DD]; SCHEMBL303180; CHEMBL4802157; GTPL12691; DTXSID101337049; NT814; EX-A6225; WHO 10952; AKOS040756249; Compound A [WO2021094247A1]; MS-31047; Example 34 [WO2007028654A1]; HY-109171; CS-0116361; 2-[3,5-bis(trifluoromethyl)phenyl]-N-{4-(4-fluoro-2-methylphenyl)-6-[(7S,9aS)-7-(hydroxymethyl)hexahydropyrazino[2,1-c][1,4]oxazin-8(1H)-yl]pyridin-3-yl}-N,2-dimethylpropanamide; BENZAMIDE, 3-(5-METHYL-2-TBENZENEACETAMIDE, N-(4-(4-FLUORO-2-METHYLPHENYL)-6-((7S,9AS)-HEXAHYDRO-7-(HYDROXYMETHYL)PYRAZINO(2,1-C)(1,4)OXAZIN-8(1H)-YL)-3-PYRIDINYL)-N,.ALPHA.,.ALPHA.-TRIMETHYL-3,5-BIS(TRIFLUOROMETHYL)-; Benzamide, 3-(5-methyl-2-tbenzeneacetamide, N-(4-(4-fluoro-2-methylphenyl)-6-((7S,9aS)-hexahydro-7-(hydroxymethyl)pyrazino(2,1-C)(1,4)oxazin-8(1H)-yl)-3-pyridinyl)-N,alpha,alpha-trimethyl-3,5-bis(trifluoromethyl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Hot flushes [ICD-11: GA30; ICD-10: N95.1] | Phase 3 | [1] | |
| Company |
Bayer
|
|||
| Structure |
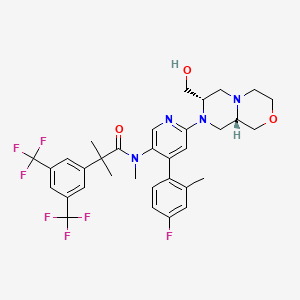 |
Download2D MOL |
||
| Formula |
C33H35F7N4O3
|
|||
| Canonical SMILES |
CC1=C(C=CC(=C1)F)C2=CC(=NC=C2N(C)C(=O)C(C)(C)C3=CC(=CC(=C3)C(F)(F)F)C(F)(F)F)N4CC5COCCN5CC4CO
|
|||
| InChI |
InChI=1S/C33H35F7N4O3/c1-19-9-23(34)5-6-26(19)27-13-29(44-16-25-18-47-8-7-43(25)15-24(44)17-45)41-14-28(27)42(4)30(46)31(2,3)20-10-21(32(35,36)37)12-22(11-20)33(38,39)40/h5-6,9-14,24-25,45H,7-8,15-18H2,1-4H3/t24-,25-/m0/s1
|
|||
| InChIKey |
DWRIJNIPBUFCQS-DQEYMECFSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Neuromedin-K receptor (TACR3) | Target Info | Antagonist | [2] |
| Substance-P receptor (TACR1) | Target Info | Antagonist | [2] | |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Measles | ||||
| Panther Pathway | CCKR signaling map ST | |||
| Reactome | G alpha (q) signalling events | |||
| WikiPathways | Gastrin-CREB signalling pathway via PKC and MAPK | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| SIDS Susceptibility Pathways | ||||
| Spinal Cord Injury | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05099159) A Double-blind, Randomized, Placebo-controlled Multicenter Study to Investigate Efficacy and Safety of Elinzanetant for the Treatment of Vasomotor Symptoms Over 26 Weeks in Postmenopausal Women. U.S.National Institutes of Health. | |||
| REF 2 | Elinzanetant (NT-814), a Neurokinin 1,3 Receptor Antagonist, Reduces Estradiol and Progesterone in Healthy Women. J Clin Endocrinol Metab. 2021 Jul 13;106(8):e3221-e3234. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

