Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DJ7Z5W
|
|||
| Drug Name |
Ro 61-8048
|
|||
| Synonyms |
199666-03-0; Ro61-8048; 3,4-dimethoxy-N-(4-(3-nitrophenyl)thiazol-2-yl)benzenesulfonamide; Ro-61-8048; 3,4-dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzenesulfonamide; CHEMBL134915; 3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-benzenesulfonamide; CHEBI:34953; C14126; MFCD11040807; 3,4-Dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulfonamide; C17H15N3O6S2; Benzenesulfonamide, 3,4-dimethoxy-N-[4-(3-nitrophenyl)-2-thiazolyl]-; AC1NQZWG; 7ZR; SCHEMBL424256; AOB1234; DTXSID20415218; EX-A805; SYN5225; HMS3886F06; BCP07470; ZINC1546077; 4013AH; BDBM50061916; s8172; AKOS024457509; CCG-222062; CS-3332; NE62855; SB19626; NCGC00370730-07; NCGC00370730-09; NCGC00370730-13; AC-32906; AK312615; AS-16581; DA-43342; HY-12347; B7334; FT-0700256; EC-000.2437; Ro 61-8048, >=98% (HPLC); J-012900; Q27116332; 3,4-Dimethoxy-N-[4-(3-nitrophenyl)-2-thiazolyl]-benzenesulfonamide; 3,4-Dimethoxy-N-[4-(3-nitrophenyl)-2-thiazolyl]benzenesulfonamide; 3,4-dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzene-1-sulfonamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Neurodegenerative disorder [ICD-11: 8A20-8A23] | Preclinical | [1] | |
| Company |
Roche
|
|||
| Structure |
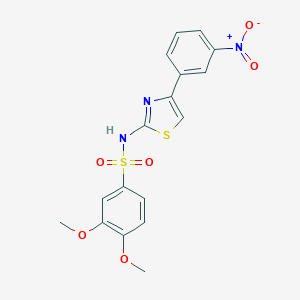 |
Download2D MOL |
||
| Formula |
C17H15N3O6S2
|
|||
| Canonical SMILES |
COC1=C(C=C(C=C1)S(=O)(=O)NC2=NC(=CS2)C3=CC(=CC=C3)[N+](=O)[O-])OC
|
|||
| InChI |
1S/C17H15N3O6S2/c1-25-15-7-6-13(9-16(15)26-2)28(23,24)19-17-18-14(10-27-17)11-4-3-5-12(8-11)20(21)22/h3-10H,1-2H3,(H,18,19)
|
|||
| InChIKey |
NDPBMCKQJOZAQX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 199666-03-0
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:34953
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Kynurenine 3-hydroxylase (KMO) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019 May;18(5):379-401. | |||
| REF 2 | Modification of kynurenine pathway via inhibition of kynurenine hydroxylase attenuates surgical brain injury complications in a male rat model. J Neurosci Res. 2020 Jan;98(1):155-167. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

