Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DI9P5G
|
|||
| Drug Name |
Orforglipron
|
|||
| Synonyms |
Orforglipron; LY3502970; 2212020-52-3; CHEMBL4446782; LY-3502970; 3-[(1S,2S)-1-(5-[(4S)-2,2-dimethyloxan-4-yl]-2-{(4S)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methyl-1H-indazol-5-yl)-2-oxo-2,3-dihydro-1H-imidazol-1-yl]-4-methyl-2,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carbonyl}-1H-indol-1-yl)-2-methylcyclopropyl]-1,2,4-oxadiazol-5(4H)-one; 3-[(1S,2S)-1-[5-[(4S)-2,2-dimethyloxan-4-yl]-2-[(4S)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methylindazol-5-yl)-2-oxoimidazol-1-yl]-4-methyl-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carbonyl]indol-1-yl]-2-methylcyclopropyl]-4H-1,2,4-oxadiazol-5-one; V6G; orforglipron [INN]; ORFORGLIPRON [USAN]; OWL833; SCHEMBL21175277; GTPL12175; 7ZW40D021M; EX-A7751; BDBM50514045; AKOS040733262; GLP-1 receptor agonist 1;Orforglipron; MS-31635; HY-112185; CS-0043632; 1,2,4-Oxadiazol-5(2H)-one, 3-[(1S,2S)-1-[2-[[(4S)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methyl-1H-indazol-5-yl)-2,3-dihydro-2-oxo-1H-imidazol-1-yl]-2,4,6,7-tetrahydro-4-methyl-5H-pyrazolo[4,3-c]pyridin-5-yl]carbonyl]-5-[(4S)-tetrahydro-2,2-dimethyl-2H-pyran-4-yl]-1H-indol-1-yl]-2-methylcyclopropyl]-; 3-[(1S,2S)-1-({2-(4-fluoro-3,5-dimethylphenyl)-3-({3-[3-(4-fluoro-1-methyl-1H-indazol-5-yl)-2-oxo-2,3-dihydro-1H-imidazol-1-yl]-4-methyl-2,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl}carbonyl)-5-[(4S)-2,2-dimethyloxan-4-yl]-1H-indol-1-yl}-2-methylcyclopropyl]-5-oxo-1,2,4-oxadiazol-4(5H)-ide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Obesity [ICD-11: 5B81; ICD-10: E66] | Phase 3 | [1] | |
| Type 2 diabetes [ICD-11: 5A11; ICD-10: E08-E13] | Phase 2 | [2] | ||
| Company |
Lilly
|
|||
| Structure |
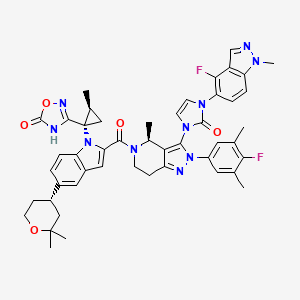 |
Download2D MOL
|
||
| Formula |
C48H48F2N10O5
|
|||
| Canonical SMILES |
CC1CC1(C2=NOC(=O)N2)N3C4=C(C=C(C=C4)C5CCOC(C5)(C)C)C=C3C(=O)N6CCC7=NN(C(=C7C6C)N8C=CN(C8=O)C9=C(C1=C(C=C9)N(N=C1)C)F)C1=CC(=C(C(=C1)C)F)C
|
|||
| InChI |
InChI=1S/C48H48F2N10O5/c1-25-18-32(19-26(2)40(25)49)60-42(58-16-15-57(46(58)63)37-11-10-36-33(41(37)50)24-51-55(36)7)39-28(4)56(14-12-34(39)53-60)43(61)38-21-31-20-29(30-13-17-64-47(5,6)23-30)8-9-35(31)59(38)48(22-27(48)3)44-52-45(62)65-54-44/h8-11,15-16,18-21,24,27-28,30H,12-14,17,22-23H2,1-7H3,(H,52,54,62)/t27-,28-,30-,48-/m0/s1
|
|||
| InChIKey |
USUWIEBBBWHKNI-KHIFEHGGSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glucagon-like peptide 1 receptor (GLP1R) | Target Info | Agonist | [3] |
| Glucagon-like peptide 1 receptor (GLP1R) | Target Info | Agonist | [4] | |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Insulin secretion | ||||
| Reactome | Glucagon-like Peptide-1 (GLP1) regulates insulin secretion | |||
| G alpha (s) signalling events | ||||
| Glucagon-type ligand receptors | ||||
| WikiPathways | GPCRs, Class B Secretin-like | |||
| Integration of energy metabolism | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05869903) A Phase 3, Randomized, Double-Blind Study to Investigate the Efficacy and Safety of Once-Daily Oral LY3502970 Compared With Placebo in Adult Participants With Obesity or Overweight With Weight-Related Comorbidities (ATTAIN-1). U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT05048719) A Phase 2 Study of Once-Daily LY3502970 Compared With Placebo and Once-Weekly Dulaglutide in Participants With Type 2 Diabetes Mellitus. U.S.National Institutes of Health. | |||
| REF 3 | Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29959-29967. | |||
| REF 4 | Orforglipron (LY3502970), a novel, oral non-peptide glucagon-like peptide-1 receptor agonist: A Phase 1b, multicentre, blinded, placebo-controlled, randomized, multiple-ascending-dose study in people with type 2 diabetes. Diabetes Obes Metab. 2023 Sep;25(9):2642-2649. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

