Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DI2KZ7
|
|||
| Drug Name |
LYS006
|
|||
| Synonyms |
LYS-006; 1799681-85-8; LTA4H-IN-1; betaS-amino-5-[4-[(5-chloro-3-fluoro-2-pyridinyl)oxy]phenyl]-2H-tetrazole-2-butanoic acid; LYS006; CHEMBL4852381; (3S)-3-amino-4-[5-[4-(5-chloro-3-fluoropyridin-2-yl)oxyphenyl]tetrazol-2-yl]butanoic acid; 59CF90960T; (3~{S})-3-azanyl-4-[5-[4-(5-chloranyl-3-fluoranyl-pyridin-2-yl)oxyphenyl]-1,2,3,4-tetrazol-2-yl]butanoic acid; SCHEMBL16827313; UNII-59CF90960T; GTPL11205; EX-A4286; BDBM50575488; MS-26579; HY-137298; CS-0137600; (S)-3-amino-4-(5-(4-((5-chloro-3-fluoropyridin-2-yl)oxy)phenyl)-2H-tetrazol-2-yl)butanoic acid; 2H-TETRAZOLE-2-BUTANOIC ACID, .BETA.-AMINO-5-(4-((5-CHLORO-3-FLUORO-2-PYRIDINYL)OXY)PHENYL)-, (.BETA.S)-; 2H-Tetrazole-2-butanoic acid, beta-amino-5-(4-((5-chloro-3-fluoro-2-pyridinyl)oxy)phenyl)-, (betaS)-; RZE
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Ulcerative colitis [ICD-11: DD71; ICD-9: 556] | Phase 2 | [1] | |
| Company |
Novartis Pharmaceuticals East Hanover, NJ
|
|||
| Structure |
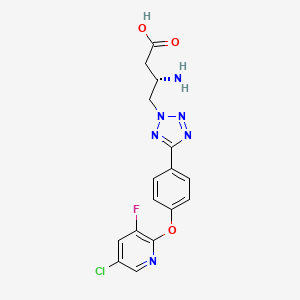 |
Download2D MOL |
||
| Formula |
C16H14ClFN6O3
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C2=NN(N=N2)CC(CC(=O)O)N)OC3=C(C=C(C=N3)Cl)F
|
|||
| InChI |
InChI=1S/C16H14ClFN6O3/c17-10-5-13(18)16(20-7-10)27-12-3-1-9(2-4-12)15-21-23-24(22-15)8-11(19)6-14(25)26/h1-5,7,11H,6,8,19H2,(H,25,26)/t11-/m0/s1
|
|||
| InChIKey |
ZEGMEJVULDALSH-NSHDSACASA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Leukotriene A-4 hydrolase (LTA4H) | Target Info | Inhibitor | [2] |
| BioCyc | Leukotriene biosynthesis | |||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Arachidonic acid metabolism | |||
| Eicosanoid Synthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04074590) A Randomized, Multi-center, Subject and Investigator-blinded, Placebo-controlled, Parallel-group Study to Assess the Efficacy Safety and Tolerability of LYS006 in Patients With Mild to Moderate Ulcerative Colitis. U.S.National Institutes of Health. | |||
| REF 2 | Discovery of LYS006, a Potent and Highly Selective Inhibitor of Leukotriene A(4) Hydrolase. J Med Chem. 2021 Feb 25;64(4):1889-1903. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

