Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DHY3Q9
|
|||
| Drug Name |
ABI-H0731
|
|||
| Synonyms |
Vebicorvir; UNII-16F6055SMG; 16F6055SMG; 11-Oxo-N-((2-(trifluoromethyl)thiazol-5-yl)methyl)-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-carboxamide 5,5-dioxide; 2090064-66-5; Vebicorvir [INN]; Vebicorvir [USAN]; SCHEMBL21250344; WHO 11201; Dibenzo(b,F)(1,4)thiazepine-8-carboxamide, 10,11-dihydro-11-oxo-N-((2-(trifluoromethyl)-5-thiazolyl)methyl)-, 5,5-dioxide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hepatitis B [ICD-11: 1E51; ICD-10: B18.1] | Phase 2 | [1] | |
| Company |
Assembly Biosciences
|
|||
| Structure |
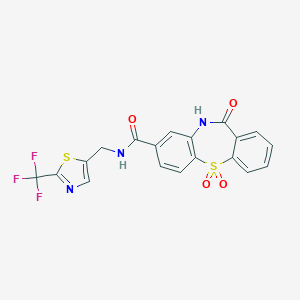 |
Download2D MOL |
||
| Formula |
C19H12F3N3O4S2
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=O)NC3=C(S2(=O)=O)C=CC(=C3)C(=O)NCC4=CN=C(S4)C(F)(F)F
|
|||
| InChI |
1S/C19H12F3N3O4S2/c20-19(21,22)18-24-9-11(30-18)8-23-16(26)10-5-6-15-13(7-10)25-17(27)12-3-1-2-4-14(12)31(15,28)29/h1-7,9H,8H2,(H,23,26)(H,25,27)
|
|||
| InChIKey |
LBJVBJYMZKEREY-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 2090064-66-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Hepatitis B virus Capsid protein (HBV C) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03576066) A Study Evaluating ABI-H0731 as Adjunctive Therapy in Participants With Chronic Hepatitis B Infection. U.S. National Institutes of Health. | |||
| REF 2 | Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020 Feb;5(2):152-166. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

