Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DH5RN1
|
|||
| Drug Name |
HQP1351
|
|||
| Synonyms |
GZD824; 1257628-77-5; GZD-824; 3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide; UNII-KV1M7Q3CBP; KV1M7Q3CBP; 4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]benzamide; CHEMBL2316582; Benzamide, 4-methyl-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]-; HQP-1351; olverembatinib; 4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}-3-(2-{1H-pyrazolo[3,4-b]pyridin-5-yl}ethynyl)benzamide; Benzamide, 4-methyl-N-(4-((4-methyl-1-piperazinyl)methyl)-3-(trifluoromethyl)phenyl)-3-(2-(1H-pyrazolo(3,4-b)pyridin-5-yl)ethynyl)-; HQP1351 free base; HQP-1351 free base; SCHEMBL3424528; GTPL10630; EX-A829; AOB87323; BCP07502; BDBM50425780; ZINC95594040; AKOS026750647; CS-1444; SB16617; compound 10a [PMID: 23301703]; NCGC00351607-06; AK547162; AS-75170; HY-15666; D-824; FT-0700150; A16264; W-6136; J-690110; 4-Methyl-N-(4-((4-methyl-1-piperazinyl)methyl)-3-(trifluoromethyl)phenyl)-3-(2-(1H-pyrazolo(3,4-b)pyridin-5-yl)ethynyl)benzamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic myeloid leukaemia [ICD-11: 2A20; ICD-10: C92.7; ICD-9: 205.1] | Phase 2 | [1] | |
| Company |
Ascentage Pharma
|
|||
| Structure |
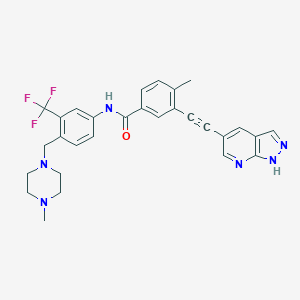 |
Download2D MOL |
||
| Formula |
C29H27F3N6O
|
|||
| Canonical SMILES |
CC1=C(C=C(C=C1)C(=O)NC2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)C#CC4=CC5=C(NN=C5)N=C4
|
|||
| InChI |
1S/C29H27F3N6O/c1-19-3-5-22(14-21(19)6-4-20-13-24-17-34-36-27(24)33-16-20)28(39)35-25-8-7-23(26(15-25)29(30,31)32)18-38-11-9-37(2)10-12-38/h3,5,7-8,13-17H,9-12,18H2,1-2H3,(H,35,39)(H,33,34,36)
|
|||
| InChIKey |
TZKBVRDEOITLRB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1257628-77-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fusion protein Bcr-Abl (Bcr-Abl) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03883100) A Pivotal Study of HQP1351 in Patients of Chronic Myeloid Leukemia in Accelerated Phase With T315I Mutation. U.S. National Institutes of Health. | |||
| REF 2 | Preclinical development of HQP1351, a multikinase inhibitor targeting a broad spectrum of mutant KIT kinases, for the treatment of imatinib-resistant gastrointestinal stromal tumors. Cell Biosci. 2019 Oct 26;9:88. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

