Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DEWC27
|
|||
| Drug Name |
Odevixibat
|
|||
| Synonyms |
501692-44-0; UNII-2W150K0UUC; 2W150K0UUC; A4250; AZD8294; 501692-44-0 (free base); Odevixibat (USAN); Odevixibat [USAN]; AR-H064974; A-4250; (S)-2-((R)-2-(2-((3,3-Dibutyl-7-(methylthio)-1,1-dioxido-5-phenyl-2,3,4,5-tetrahydrobenzo[f][1,2,5]thiadiazepin-8-yl)oxy)acetamido)-2-(4-hydroxyphenyl)acetamido)butanoic acid; SCHEMBL946468; CHEMBL4297588; BDBM77040; GTPL11194; AZD-8294; example 5 [US9694018B1]; WHO 10706; HY-109120; Unk-D-nTyr-Abu-OH (IUPAC condensed name); CS-0078340; D11716; US9694018, 5; (2S)-2-(((2R)-2-((((3,3-Dibutyl-7-(methylthio)-1,1-dioxido-5-phenyl-2,3,4,5-tetrahydro- 1,2,5-benzothiadiazepin-8-yl)oxy)acetyl)amino)-2-(4-hydroxyphenyl)acetyl)amino)butanoic acid; (2S)-2-[[(2R)-2-[[2-[(3,3-dibutyl-7-methylsulfanyl-1,1-dioxo-5-phenyl-2,4-dihydro-1lambda6,2,5-benzothiadiazepin-8-yl)oxy]acetyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]butanoic acid; Butanoic acid, 2-(((2R)-((((3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl)oxy)acetyl)amino)(4-hydroxyphenyl)acetyl)amino)-, (2S)-; Butanoic acid, 2-(((2R)-2-((2-((3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,2,5-benzothiadiazepin-8-yl)oxy)acetyl)amino)-2-(4-hydroxyphenyl)acetyl)amino)-, (2S)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Pruritus [ICD-11: EC90; ICD-10: L29, L29.9; ICD-9: 698] | Approved | [1] | |
| Alagille syndrome [ICD-11: LB20.0Y] | Phase 3 | [2] | ||
| Company |
Albireo Pharma
|
|||
| Structure |
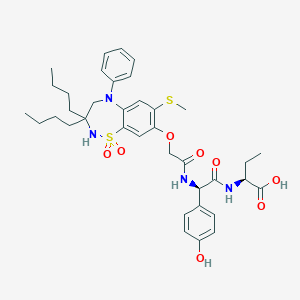 |
Download2D MOL
|
||
| Formula |
C37H48N4O8S2
|
|||
| Canonical SMILES |
CCCCC1(CN(C2=CC(=C(C=C2S(=O)(=O)N1)OCC(=O)NC(C3=CC=C(C=C3)O)C(=O)NC(CC)C(=O)O)SC)C4=CC=CC=C4)CCCC
|
|||
| InChI |
1S/C37H48N4O8S2/c1-5-8-19-37(20-9-6-2)24-41(26-13-11-10-12-14-26)29-21-31(50-4)30(22-32(29)51(47,48)40-37)49-23-33(43)39-34(25-15-17-27(42)18-16-25)35(44)38-28(7-3)36(45)46/h10-18,21-22,28,34,40,42H,5-9,19-20,23-24H2,1-4H3,(H,38,44)(H,39,43)(H,45,46)/t28-,34+/m0/s1
|
|||
| InChIKey |
XULSCZPZVQIMFM-IPZQJPLYSA-N
|
|||
| CAS Number |
CAS 501692-44-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Ileal sodium/bile acid cotransporter (SLC10A2) | Target Info | Inhibitor | [3] |
| KEGG Pathway | Bile secretion | |||
| Reactome | Recycling of bile acids and salts | |||
| WikiPathways | Bile acid and bile salt metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 215498. | |||
| REF 2 | ClinicalTrials.gov (NCT04674761) Efficacy and Safety of Odevixibat in Patients With Alagille Syndrome (ASSERT). U.S. National Institutes of Health. | |||
| REF 3 | Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol Int. 2020 Sep;14(5):677-689. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

