Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DE53JQ
|
|||
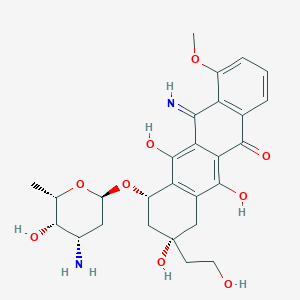
| Drug Name |
Camsirubicin
|
|||
| Synonyms |
UNII-VI79RD8VNN; VI79RD8VNN; 236095-26-4; (8R,10S)-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyethyl)-12-imino-1-methoxy-7,9,10,12-tetrahydrotetracen-5(8H)-one; 5(8H)-Naphthacenone, 10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,9,10,12-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyethyl)-12-imino-1-methoxy-, (8R,10S)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Soft tissue sarcoma [ICD-11: 2B57; ICD-9: 171] | Phase 2 | [1] | |
| Company |
Monopar Therapeutics
|
|||
| Structure |
 |
Download2D MOL |
||
| Formula |
C27H32N2O9
|
|||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=N)C(=CC=C5)OC)O)(CCO)O)N)O
|
|||
| InChI |
1S/C27H32N2O9/c1-11-23(31)14(28)8-17(37-11)38-16-10-27(35,6-7-30)9-13-19(16)26(34)20-21(25(13)33)24(32)12-4-3-5-15(36-2)18(12)22(20)29/h3-5,11,14,16-17,23,29-31,33-35H,6-10,28H2,1-2H3/t11-,14-,16-,17-,23+,27-/m0/s1
|
|||
| InChIKey |
GNCWGPLZJLZZPI-KUIJCEFOSA-N
|
|||
| CAS Number |
CAS 236095-26-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II alpha (TOP2A) | Target Info | Inhibitor | [2] |
| NetPath Pathway | TCR Signaling Pathway | |||
| IL4 Signaling Pathway | ||||
| Panther Pathway | DNA replication | |||
| Pathway Interaction Database | Validated transcriptional targets of deltaNp63 isoforms | |||
| Reactome | G0 and Early G1 | |||
| WikiPathways | Retinoblastoma (RB) in Cancer | |||
| Integrated Pancreatic Cancer Pathway | ||||
| Gastric cancer network 2 | ||||
| Gastric Cancer Network 1 | ||||
| Mitotic G1-G1/S phases | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of Monopar Therapeutics. | |||
| REF 2 | Clinical pipeline report, company report or official report of Monopar Therapeutics. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

