Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DE3VU1
|
|||
| Drug Name |
Branebrutinib
|
|||
| Synonyms |
Branebrutinib; BMS-986195; 1912445-55-6; (S)-4-(3-(but-2-ynamido)piperidin-1-yl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 7LBRZUYSHU; Branebrutinib [USAN]; BMS986195; 4-[(3S)-3-(but-2-ynoylamino)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; Branebrutinib (USAN); 1H-Indole-7-carboxamide, 5-fluoro-2,3-dimethyl-4-((3S)-3-((1-oxo-2-butyn-1-yl)amino)-1-piperidinyl)-; 4-((3S)-3-(But-2-ynamido)piperidin-1-yl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 4-[(3S)-3-(but-2-ynamido)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; UNII-7LBRZUYSHU; BRANEBRUTINIB [INN]; BRANEBRUTINIB [WHO-DD]; GTPL9869; CHEMBL4297674; SCHEMBL17699728; Branebrutinib (BMS-986195); C20H23FN4O2; VJPPLCNBDLZIFG-ZDUSSCGKSA-N; BDBM164638; BDBM166759; BCP29496; EX-A2720; MFCD31631584; NSC807627; s8832; WHO 11026; AKOS037649047; DB15347; NSC-807627; AC-31535; BS-16393; BMS986195; BMS986195; HY-112161; CS-0043577; Example 223 [US20160115126A1]; D11478; EN300-2007801; US9688629, 123; US9688629, 223; Q50825082; 4-((3S)-3-(2-Butynoylamino)-1-piperidinyl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; (S)-4-(3-(2-BUTYNOYLAMINO)PIPERIDIN-1-YL)-5-FLUORO-2,3-DIMETHYL-1H-INDOLE-7-CARBOXAMIDE; 4-((3S)-3-(2-BUTYNOYLAMINO)-1-PIPERIDINYL)-5-FLUORO-2,3-DIMETHYL-1HINDOLE-7-CARBOXAMIDE
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Rheumatoid arthritis [ICD-11: FA20; ICD-9: 729] | Phase 2 | [1] | |
| Company |
Bristol-Myers Squibb Princeton, NJ
|
|||
| Structure |
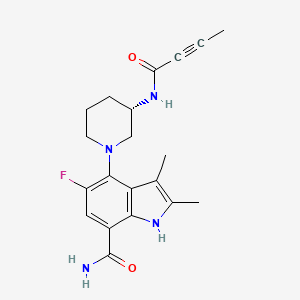 |
Download2D MOL |
||
| Formula |
C20H23FN4O2
|
|||
| Canonical SMILES |
CC#CC(=O)NC1CCCN(C1)C2=C(C=C(C3=C2C(=C(N3)C)C)C(=O)N)F
|
|||
| InChI |
InChI=1S/C20H23FN4O2/c1-4-6-16(26)24-13-7-5-8-25(10-13)19-15(21)9-14(20(22)27)18-17(19)11(2)12(3)23-18/h9,13,23H,5,7-8,10H2,1-3H3,(H2,22,27)(H,24,26)/t13-/m0/s1
|
|||
| InChIKey |
VJPPLCNBDLZIFG-ZDUSSCGKSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04186871) A Randomized, Placebo-Controlled, Double-Blind, Multicenter Study to Assess the Efficacy and Safety of Branebrutinib Treatment in Subjects With Active Systemic Lupus Erythematosus or Primary Sj?gren's Syndrome, or Branebrutinib Treatment Followed by Open-label Abatacept Treatment in Subjects With Active Rheumatoid Arthritis. U.S.National Institutes of Health. | |||
| REF 2 | Safety, pharmacokinetics and pharmacodynamics of branebrutinib (BMS-986195), a covalent, irreversible inhibitor of Bruton's tyrosine kinase: Randomised phase I, placebo-controlled trial in healthy participants. Br J Clin Pharmacol. 2020 Sep;86(9):1849-1859. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

