Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DCT2Q3
|
|||
| Drug Name |
Annamycin
|
|||
| Synonyms |
92689-49-1; UNII-SNU299M83Q; 2'-Iodo-3'-hydroxy-4'-epi-4-demethoxydoxorubicin; SNU299M83Q; (7S,9S)-7-(((2R,3R,4R,5R,6S)-4,5-Dihydroxy-3-iodo-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7,8,9,10-tetrahydrotetracene-5,12-dione; AR-522; SCHEMBL19368; (7S,9S)-7-[(2R,3R,4R,5R,6S)-4,5-dihydroxy-3-iodo-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-8,10-dihydro-7H-tetracene-5,12-dione; ZINC3918134; DB06420; 5,12-Naphthacenedione, 7-((2,6-dideoxy-2-iodo-alpha-L-mannopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-9-(hydroxyacetyl)-, (7S-cis)-; 689A491; Q4767903
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60] | Phase 1/2 | [1] | |
| Company |
Moleculin Biotech
|
|||
| Structure |
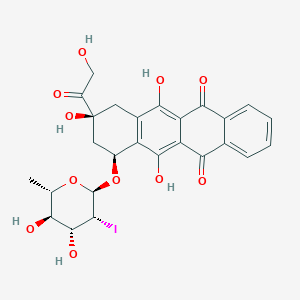 |
Download2D MOL |
||
| Formula |
C26H25IO11
|
|||
| Canonical SMILES |
CC1C(C(C(C(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=CC=CC=C5C4=O)O)(C(=O)CO)O)I)O)O
|
|||
| InChI |
1S/C26H25IO11/c1-9-19(30)24(35)18(27)25(37-9)38-13-7-26(36,14(29)8-28)6-12-15(13)23(34)17-16(22(12)33)20(31)10-4-2-3-5-11(10)21(17)32/h2-5,9,13,18-19,24-25,28,30,33-36H,6-8H2,1H3/t9-,13-,18+,19-,24-,25-,26-/m0/s1
|
|||
| InChIKey |
CIDNKDMVSINJCG-GKXONYSUSA-N
|
|||
| CAS Number |
CAS 92689-49-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | DNA topoisomerase II alpha (TOP2A) | Target Info | . | [2] |
| NetPath Pathway | TCR Signaling Pathway | |||
| IL4 Signaling Pathway | ||||
| Panther Pathway | DNA replication | |||
| Pathway Interaction Database | Validated transcriptional targets of deltaNp63 isoforms | |||
| Reactome | G0 and Early G1 | |||
| WikiPathways | Retinoblastoma (RB) in Cancer | |||
| Integrated Pancreatic Cancer Pathway | ||||
| Gastric cancer network 2 | ||||
| Gastric Cancer Network 1 | ||||
| Mitotic G1-G1/S phases | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Effect of structural modification at the 4, 3', and 2' positions of doxorubicin on topoisomerase II poisoning, apoptosis, and cytotoxicity in human melanoma cells. Arch Immunol Ther Exp (Warsz). May-Jun 2007;55(3):193-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

