Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DCA5M2
|
|||
| Drug Name |
Padsevonil
|
|||
| Synonyms |
1294000-61-5; UNII-0R1HN52K0N; UCB0942; UCB1415943-000; (R)-4-(2-chloro-2,2-difluoroethyl)-1-((2-(methoxymethyl)-6-(trifluoromethyl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl)methyl)pyrrolidin-2-one; (4R)-4-(2-Chloro-2,2-difluoroethyl)-1-((2-(methoxymethyl)-6-(trifluoromethyl)imidazo(2,1-b)(1,3,4)thiadiazol-5-yl)methyl)pyrrolidin-2-one; (4R)-4-(2-chloro-2,2-difluoroethyl)-1-[[2-(methoxymethyl)-6-(trifluoromethyl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl]methyl]pyrrolidin-2-one; Padsevonil [INN]; Padsevonil [USAN]; Padsevonil (JAN/USAN); SCHEMBL1672843; CHEMBL4297521; UCB-0942; WHO 10384; DB14977; SB18725; HY-109009; CS-0030507; D11842; Q27237119; (4R)-4-(2-Chloro-2,2-difluoroethyl)-1-((2-(methoxymethyl)-6-(trifluoromethyl)imidazo(2,1-b)(1,3,4)thiadiazol-5-yl)methyl)-2-pyrrolidinone; 2-Pyrrolidinone, 4-(2-chloro-2,2-difluoroethyl)-1-((2-(methoxymethyl)-6-(trifluoromethyl)imidazo(2,1-b)-1,3,4-thiadiazol-5-yl)methyl)-, (4R)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68] | Phase 2/3 | [1] | |
| Company |
UCB
|
|||
| Structure |
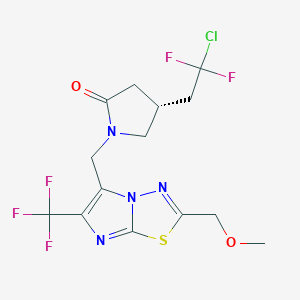 |
Download2D MOL |
||
| Formula |
C14H14ClF5N4O2S
|
|||
| Canonical SMILES |
COCC1=NN2C(=C(N=C2S1)C(F)(F)F)CN3CC(CC3=O)CC(F)(F)Cl
|
|||
| InChI |
1S/C14H14ClF5N4O2S/c1-26-6-9-22-24-8(11(14(18,19)20)21-12(24)27-9)5-23-4-7(2-10(23)25)3-13(15,16)17/h7H,2-6H2,1H3/t7-/m1/s1
|
|||
| InChIKey |
DCXFIOLWWRXEQH-SSDOTTSWSA-N
|
|||
| CAS Number |
CAS 1294000-61-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | GABA(A) receptor (GABAR) | Target Info | Inhibitor | [2] |
| Synaptic vesicle glycoprotein 2A (SV2A) | Target Info | Inhibitor | [2] | |
| KEGG Pathway | ECM-receptor interaction | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03370120) Study to Test the Safety and Efficacy of Padsevonil as Adjunctive Treatment of Focal-onset Seizures in Adult Subjects With Drug-resistant Epilepsy. U.S. National Institutes of Health. | |||
| REF 2 | Pharmacological Profile of the Novel Antiepileptic Drug Candidate Padsevonil: Characterization in Rodent Seizure and Epilepsy Models. J Pharmacol Exp Ther. 2020 Jan;372(1):11-20. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

