Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D9YG4P
|
|||
| Drug Name |
APX3330
|
|||
| Synonyms |
E3330; 136164-66-4; E-3330; E 3330; CHEMBL578390; UNII-11267UI968; 11267UI968; (2E)-2-[(4,5-dimethoxy-2-methyl-3,6-dioxocyclohexa-1,4-dien-1-yl)methylidene]undecanoic acid; (E)-2-((4,5-dimethoxy-2-methyl-3,6-dioxocyclohexa-1,4-dien-1-yl)methylene)undecanoic acid; (2E)-3-(5-(2,3-Dimethoxy-6-methyl-1,4-benzoquinoyl))-2-nonyl-2-propenoic acid; SCHEMBL3758716; SCHEMBL3758719; HMS3886N15; APX 3330; EX-A2212; BDBM50303955; s7445; ZINC14252145; CCG-268389; HY-19357; B5875; CS-0015424; E3330, >=98% (HPLC); A14440; (E)-3-(4,5-dimethoxy-2-methyl-3,6-dioxocyclohexa-1,4-dienyl)-2-nonylpropenoic acid; (E)-3-(5,6-Dimethoxy-3-methyl-14-dioxocyclohexa-25-dienyl)-2-nonylpropenoic Acid; Undecanoic acid, 2-((4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)methylene)-, (E)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Diabetic retinopathy [ICD-11: 9B71.0; ICD-10: H36.0] | Phase 2 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199] | Phase 1 | [2] | ||
| Company |
Apexian Pharmaceuticals
|
|||
| Structure |
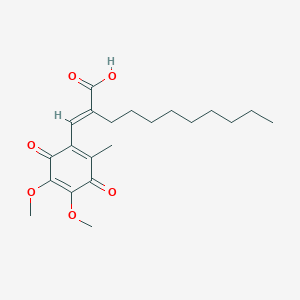 |
Download2D MOL |
||
| Formula |
C21H30O6
|
|||
| Canonical SMILES |
CCCCCCCCCC(=CC1=C(C(=O)C(=C(C1=O)OC)OC)C)C(=O)O
|
|||
| InChI |
1S/C21H30O6/c1-5-6-7-8-9-10-11-12-15(21(24)25)13-16-14(2)17(22)19(26-3)20(27-4)18(16)23/h13H,5-12H2,1-4H3,(H,24,25)/b15-13+
|
|||
| InChIKey |
AALSSIXXBDPENJ-FYWRMAATSA-N
|
|||
| CAS Number |
CAS 136164-66-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | AP endonuclease 1 (APEX1) | Target Info | Inhibitor | [3] |
| KEGG Pathway | Base excision repair | |||
| Pathway Interaction Database | HIF-2-alpha transcription factor network | |||
| WikiPathways | Spinal Cord Injury | |||
| TSH signaling pathway | ||||
| Base Excision Repair | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04692688) Study of the Safety and Efficacy of APX3330 in Diabetic Retinopathy (ZETA-1). U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT03375086) A Study of APX3330 in Patients With Advanced Solid Tumors (APX3330). U.S. National Institutes of Health. | |||
| REF 3 | Exploiting the Ref-1-APE1 node in cancer signaling and other diseases: from bench to clinic. NPJ Precis Oncol. 2017;1:19. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

