Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D9UGX7
|
|||
| Drug Name |
Encequidar
|
|||
| Synonyms |
849675-66-7; HM-30181-A; HM-30181; HM30181; UNII-K4I4I996O4; K4I4I996O4; 849675-66-7 (free base); HM 30181A; N-(2-(2-(4-(2-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)phenyl)-2H-tetrazol-5-yl)-4,5-dimethoxyphenyl)-4-oxo-4H-chromene-2-carboxamide; HM30181A; Encequidar [USAN]; HM-30181A; Encequidar (USAN/INN); HM-30181 free base; CHEMBL4594298; SCHEMBL13822558; EX-A3429A; BCP25240; MFCD25976625; WHO 10861; ZINC68014383; CS-6194; DB14070; SB18921; 4-Oxo-4H-chromene-2-carboxylic acid (2-(2-4-(2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl)-phenyl-2H-tetrazol-5-yl)-4,5-dimethoxy-phenyl)-amide; AS-35283; HY-13646; D11782; Q27281950; 4-oxo-4H-chromen-2-carboxylic acid [2-(2-{4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-ethyl]-phenyl}-2H-tetrazol-5-yl)-4,5-dimethoxy-phenyl]-amide; 4H-1-Benzopyran-2-carboxamide, N-[2-[2-[4-[2-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)ethyl]phenyl]-2H-tetrazol-5-yl]-4,5-dimethoxyphenyl]-4-oxo-; N-(2-(2-(4-(2-(6,7-dimethoxy-3,4-dihydroisoquinoline-2(1H)-yl)ethyl)phenyl)-2H-tetrazol-5-yl)-4,5-dimethoxyphenyl)-4-oxo-4H-chromene-2-carboxamide; N-[2-[2-[4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]tetrazol-5-yl]-4,5-dimethoxyphenyl]-4-oxochromene-2-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Metastatic breast cancer [ICD-11: 2C6Y] | Phase 2 | [1] | |
| Company |
Athenex Oncology
|
|||
| Structure |
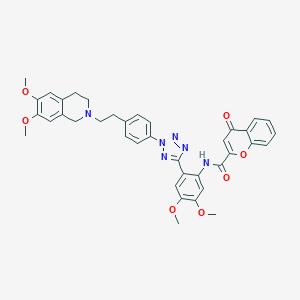 |
Download2D MOL
|
||
| Formula |
C38H36N6O7
|
|||
| Canonical SMILES |
COC1=C(C=C2CN(CCC2=C1)CCC3=CC=C(C=C3)N4N=C(N=N4)C5=CC(=C(C=C5NC(=O)C6=CC(=O)C7=CC=CC=C7O6)OC)OC)OC
|
|||
| InChI |
1S/C38H36N6O7/c1-47-32-17-24-14-16-43(22-25(24)18-33(32)48-2)15-13-23-9-11-26(12-10-23)44-41-37(40-42-44)28-19-34(49-3)35(50-4)20-29(28)39-38(46)36-21-30(45)27-7-5-6-8-31(27)51-36/h5-12,17-21H,13-16,22H2,1-4H3,(H,39,46)
|
|||
| InChIKey |
AHJUHHDDCJQACA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 849675-66-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Multidrug resistance protein 3 (ABCB4) | Target Info | Inhibitor | [2] |
| KEGG Pathway | ABC transporters | |||
| Bile secretion | ||||
| Reactome | PPARA activates gene expression | |||
| ABC-family proteins mediated transport | ||||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | |||
| Farnesoid X Receptor Pathway | ||||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01042379) I-SPY TRIAL: Neoadjuvant and Personalized Adaptive Novel Agents to Treat Breast Cancer (I-SPY). U.S. National Institutes of Health. | |||
| REF 2 | Discovery of Encequidar, First-in-Class Intestine Specific P-glycoprotein Inhibitor. J Med Chem. 2021 Apr 8;64(7):3677-3693. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

