Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D8FHG5
|
|||
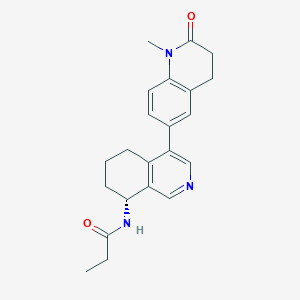
| Drug Name |
Baxdrostat
|
|||
| Synonyms |
Baxdrostat; Baxdrostat [INN]; NF3P9Z8J5Y; 1428652-17-8; UNII-NF3P9Z8J5Y; (+)-(R)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide; N-((8R)-5,6,7,8-Tetrahydro-4-(1,2,3,4-tetrahydro-1-methyl-2-oxo-6-quinolinyl)-8-isoquinolinyl)propanamide; Propanamide, N-((8R)-5,6,7,8-tetrahydro-4-(1,2,3,4-tetrahydro-1-methyl-2-oxo-6-quinolinyl)-8-isoquinolinyl)-; CIN107; CHEMBL4113975; SCHEMBL14799753; GTPL12362; CIN-107; BDBM233806; GLXC-26998; MS-25786; HY-132809; RO6836191; CS-0204056; US9353081, 3-1; N-[(8R)-4-(1-methyl-2-oxo-3,4-dihydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl]propanamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Phase 2 | [1] | |
| Company |
CinCor Pharma
|
|||
| Structure |
 |
Download2D MOL |
||
| Formula |
C22H25N3O2
|
|||
| Canonical SMILES |
CCC(=O)NC1CCCC2=C1C=NC=C2C3=CC4=C(C=C3)N(C(=O)CC4)C
|
|||
| InChI |
InChI=1S/C22H25N3O2/c1-3-21(26)24-19-6-4-5-16-17(12-23-13-18(16)19)14-7-9-20-15(11-14)8-10-22(27)25(20)2/h7,9,11-13,19H,3-6,8,10H2,1-2H3,(H,24,26)/t19-/m1/s1
|
|||
| InChIKey |
VDEUDSRUMNAXJG-LJQANCHMSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Aldosterone synthase (CYP11B2) | Target Info | Inhibitor | [2] |
| BioCyc | Superpathway of steroid hormone biosynthesis | |||
| Mineralocorticoid biosynthesis | ||||
| KEGG Pathway | Steroid hormone biosynthesis | |||
| Metabolic pathways | ||||
| Pathwhiz Pathway | Steroidogenesis | |||
| Reactome | Glucocorticoid biosynthesis | |||
| Endogenous sterols | ||||
| WikiPathways | Metapathway biotransformation | |||
| ACE Inhibitor Pathway | ||||
| Oxidation by Cytochrome P450 | ||||
| Metabolism of steroid hormones and vitamin D | ||||
| Glucocorticoid & Mineralcorticoid Metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. N Engl J Med. 2023 Feb 2;388(5):395-405. | |||
| REF 2 | Phase 2 Trial of Baxdrostat for Treatment-Resistant Hypertension. N Engl J Med. 2023 Feb 2;388(5):395-405. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

