Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D87IQS
|
|||
| Drug Name |
Mitiperstat
|
|||
| Synonyms |
Mitiperstat; AZD4831; Mitiperstat [INN]; AZD-4831; S6GYK3X4QQ; 1933460-19-5; UNII-S6GYK3X4QQ; AZD-4831 [WHO-DD]; 1-((2-((1R)-1-Aminoethyl)-4-chloro-phenyl)methyl)-2-thioxo-5hpyrrolo(3,2-d)pyrimidin-4-one; 4H-Pyrrolo(3,2-d)pyrimidin-4-one, 1-((2-((1R)-1-aminoethyl)-4-chlorophenyl)methyl)-1,2,3,5-tetrahydro-2-thioxo-; 4H-Pyrrolo[3,2-d]pyrimidin-4-one, 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-1,2,3,5-tetrahydro-2-thioxo-; Alternative Preparation; MITIPERSTAT [USAN]; Azd 4831; CHEMBL5095218; SCHEMBL17782047; GTPL12154; AZD 4831 [WHO-DD]; BHKKSKOHRFHHIN-MRVPVSSYSA-N; BDBM312172; GLXC-26157; EX-A7129; compound 16 [PMID: 36005476]; HY-145581; CS-0376445; US9616063, 3; 1-({2-[(1R)-1-aminoethyl]-4-chlorophenyl}methyl)-2-sulfanylidene-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one; 1-({2-[(1R)-1-aminoethyl]-4-chlorophenyl}methyl)-2-sulfanylidene-1H,2H,3H,4H,5H-pyrrolo[3,2-d]pyrimidin-4-one; 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-2-sulfanylidene-5H-pyrrolo[3,2-d]pyrimidin-4-one; 1-{2-[(1R)-1-Aminoethyl]-4-chlorobenzyl}-2-thioxo-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Heart failure with preserved ejection fraction [ICD-11: BD11.0] | Phase 2/3 | [1] | |
| Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J40-J44, J47; ICD-9: 490-492, 494-496] | Phase 2 | [2] | ||
| Company |
AstraZeneca
|
|||
| Structure |
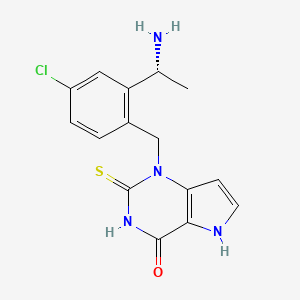 |
Download2D MOL |
||
| Formula |
C15H15ClN4OS
|
|||
| Canonical SMILES |
CC(C1=C(C=CC(=C1)Cl)CN2C3=C(C(=O)NC2=S)NC=C3)N
|
|||
| InChI |
InChI=1S/C15H15ClN4OS/c1-8(17)11-6-10(16)3-2-9(11)7-20-12-4-5-18-13(12)14(21)19-15(20)22/h2-6,8,18H,7,17H2,1H3,(H,19,21,22)/t8-/m1/s1
|
|||
| InChIKey |
BHKKSKOHRFHHIN-MRVPVSSYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Myeloperoxidase (MPO) | Target Info | Inhibitor | [3] |
| KEGG Pathway | Phagosome | |||
| Transcriptional misregulation in cancer | ||||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| IL23-mediated signaling events | ||||
| WikiPathways | Folate Metabolism | |||
| Vitamin B12 Metabolism | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04986202) A Randomised, Double-blind, Placebo-controlled, Multi-center Sequential Phase 2b and Phase 3 Study to Evaluate the Efficacy and Safety of AZD4831 Administered for Up to 48 Weeks in Participants With Heart Failure With Left Ventricular Ejection Fraction > 40%. U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT05492877) A Phase IIa Randomised, Double Blind, Placebo Controlled, Parallel Arm, Multi-Centre Study to Evaluate the Efficacy and Safety of Mitiperstat (AZD4831), for 12-24 Weeks, in Patients With Moderate to Severe Chronic Obstructive Pulmonary Disease (COPD). U.S.National Institutes of Health. | |||
| REF 3 | Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction. J Med Chem. 2022 Sep 8;65(17):11485-11496. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

