Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D7M3WV
|
|||
| Drug Name |
Dersimelagon
|
|||
| Synonyms |
Dersimelagon; 1835256-48-8; Dersimelagon [INN]; Dersimelagon [USAN]; MT-7117; 1CWH5SV4G2; 1-(2-((3S,4R)-1-((3R,4R)-1-Cyclopentyl-3-fluoro-4-(4-methoxyphenyl)pyrrolidine-3-carbonyl)-4-(methoxymethyl)pyrrolidin-3-yl)-5-(trifluoromethyl)phenyl)piperidine-4-carboxylic acid; Dersimelagon [USAN:INN]; UNII-1CWH5SV4G2; DERSIMELAGON [WHO-DD]; CHEMBL4802160; SCHEMBL18686769; MUNWOYRHJPWQNE-GMFUQMJFSA-N; WHO 10832; AKOS040746751; AC-36942; HY-109114; CS-0077733; 4-PIPERIDINECARBOXYLIC ACID, 1-(2-((3S,4R)-1-(((3R,4R)-1-CYCLOPENTYL-3-FLUORO-4-(4-METHOXYPHENYL)-3-PYRROLIDINYL)CARBONYL)-4-(METHOXYMETHYL)-3-PYRROLIDINYL)-5- (TRIFLUOROMETHYL)PHENYL)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Erythropoietic protoporphyria [ICD-11: 5C58.12] | Phase 3 | [1] | |
| Company |
Mitsubishi Tanabe Pharma
|
|||
| Structure |
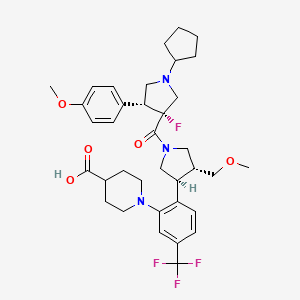 |
Download2D MOL |
||
| Formula |
C36H45F4N3O5
|
|||
| Canonical SMILES |
COCC1CN(CC1C2=C(C=C(C=C2)C(F)(F)F)N3CCC(CC3)C(=O)O)C(=O)C4(CN(CC4C5=CC=C(C=C5)OC)C6CCCC6)F
|
|||
| InChI |
InChI=1S/C36H45F4N3O5/c1-47-21-25-18-42(19-30(25)29-12-9-26(36(38,39)40)17-32(29)41-15-13-24(14-16-41)33(44)45)34(46)35(37)22-43(27-5-3-4-6-27)20-31(35)23-7-10-28(48-2)11-8-23/h7-12,17,24-25,27,30-31H,3-6,13-16,18-22H2,1-2H3,(H,44,45)/t25-,30+,31+,35+/m1/s1
|
|||
| InChIKey |
MUNWOYRHJPWQNE-GMFUQMJFSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Melanocortin receptor 1 (MC1R) | Target Info | Agonist | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Melanogenesis | ||||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (s) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04402489) A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Efficacy, Safety, and Tolerability of MT-7117 in Adults and Adolescents With Erythropoietic Protoporphyria or X-Linked Protoporphyria. U.S.National Institutes of Health. | |||
| REF 2 | Dersimelagon, a novel oral melanocortin 1 receptor agonist, demonstrates disease-modifying effects in preclinical models of systemic sclerosis. Arthritis Res Ther. 2022 Sep 1;24(1):210. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

