Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D5U4MY
|
|||
| Drug Name |
Etavopivat
|
|||
| Synonyms |
Etavopivat; FT-4202; Etavopivat [INN]; Etavopivat [USAN]; 2245053-57-8; V4E0A9M44Q; 1-Propanone, 1-(5-((2,3-dihydro-1,4-dioxino(2,3-b)pyridin-7-yl)sulfonyl)-3,4,5,6-tetrahydropyrrolo(3,4-C)pyrrol-2(1H)-yl)-3-hydroxy-2-phenyl-, (2S)-; 1-Propanone, 1-[5-[(2,3-dihydro-1,4-dioxino[2,3-b]pyridin-7-yl)sulfonyl]-3,4,5,6-tetrahydropyrrolo[3,4-c]pyrrol-2(1H)-yl]-3-hydroxy-2-phenyl-, (2S)-; Etavopivat [USAN:INN]; UNII-V4E0A9M44Q; CHEMBL4650332; SCHEMBL20511240; GTPL11975; GLXC-25164; EX-A6335; FT4202; WHO 11646; AKOS040757470; MS-28351; CS-0213556; (2S)-1-(5-(2,3-Dihydro(1,4)dioxino(2,3-b)pyridine-7- sulfonyl)-3,4,5,6-tetrahydropyrrolo(3,4-C)pyrrol-2(1H)- YL)-3-hydroxy-2-phenylpropan-1-one; (2S)-1-[5-(2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-7-ylsulfonyl)-1,3,4,6-tetrahydropyrrolo[3,4-c]pyrrol-2-yl]-3-hydroxy-2-phenylpropan-1-one; (S)-1-(5-((2,3-dihydro-[1,4]dioxino[2,3-b]pyridin-7-yl)sulfonyl)-3,4,5,6-tetrahydropyrrolo[3,4-c]pyrrol-2(1H)-yl)-3-hydroxy-2-phenylpropan-1-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Sickle-cell disorder [ICD-11: 3A51; ICD-10: D57, D57.8; ICD-9: 282.5, 282.6] | Phase 2/3 | [1] | |
| Company |
Forma Therapeutics
|
|||
| Structure |
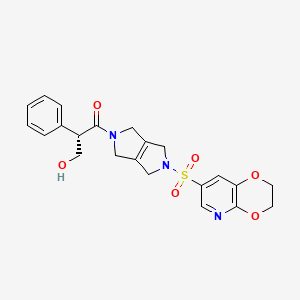 |
Download2D MOL |
||
| Formula |
C22H23N3O6S
|
|||
| Canonical SMILES |
C1COC2=C(O1)C=C(C=N2)S(=O)(=O)N3CC4=C(C3)CN(C4)C(=O)C(CO)C5=CC=CC=C5
|
|||
| InChI |
InChI=1S/C22H23N3O6S/c26-14-19(15-4-2-1-3-5-15)22(27)24-10-16-12-25(13-17(16)11-24)32(28,29)18-8-20-21(23-9-18)31-7-6-30-20/h1-5,8-9,19,26H,6-7,10-14H2/t19-/m1/s1
|
|||
| InChIKey |
KZFFYEPYCVDOGE-LJQANCHMSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Pyruvate kinase (PK) | Target Info | . | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04624659) An Adaptive, Randomized, Placebo-controlled, Double-blind, Multi-center Study of Oral Etavopivat, a Pyruvate Kinase Activator in Patients With Sickle Cell Disease (HIBISCUS). U.S.National Institutes of Health. | |||
| REF 2 | Etavopivat, a Pyruvate Kinase Activator in Red Blood Cells, for the Treatment of Sickle Cell Disease. J Pharmacol Exp Ther. 2022 Mar;380(3):210-219. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

