Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D5SDZ0
|
|||
| Drug Name |
RPT193
|
|||
| Synonyms |
RPT193; Zelnecirnon; 2366152-15-8; zelnecirnon [INN]; RPT193; Zelnecirnon; Q0M1LOC2MM; SCHEMBL21193871; SCHEMBL21207415; GTPL12893; RPT-193; GLXC-26793; example 37 [WO2019147862A1]; MS-30263; HY-148074; CS-0610200; (1R,3r)-3-((R)-3-(1-(5-Chloro-4-(((R)-1-(2,4-dichlorophenyl)ethyl)amino)-6-methylpyrimidin-2-yl)azetidin-3-yl)piperidin-1-yl)-1-methylcyclobutane-1-carboxylic acid; 3-[(3R)-3-[1-[5-chloro-4-[[(1R)-1-(2,4-dichlorophenyl)ethyl]amino]-6-methylpyrimidin-2-yl]azetidin-3-yl]piperidin-1-yl]-1-methylcyclobutane-1-carboxylic acid; Cyclobutanecarboxylic acid, 3-[(3R)-3-[1-[5-chloro-4-[[(1R)-1-(2,4-dichlorophenyl)ethyl]amino]-6-methyl-2-pyrimidinyl]-3-azetidinyl]-1-piperidinyl]-1-methyl-, trans-
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Phase 2 | [1] | |
| Company |
RAPT Therapeutics South San Francisco, CA
|
|||
| Structure |
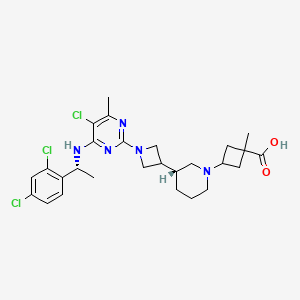 |
Download2D MOL |
||
| Formula |
C27H34Cl3N5O2
|
|||
| Canonical SMILES |
CC1=C(C(=NC(=N1)N2CC(C2)C3CCCN(C3)C4CC(C4)(C)C(=O)O)NC(C)C5=C(C=C(C=C5)Cl)Cl)Cl
|
|||
| InChI |
InChI=1S/C27H34Cl3N5O2/c1-15(21-7-6-19(28)9-22(21)29)31-24-23(30)16(2)32-26(33-24)35-13-18(14-35)17-5-4-8-34(12-17)20-10-27(3,11-20)25(36)37/h6-7,9,15,17-18,20H,4-5,8,10-14H2,1-3H3,(H,36,37)(H,31,32,33)/t15-,17+,20?,27?/m1/s1
|
|||
| InChIKey |
ANPSFQZLPNWKHR-PRZTZBHNSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | C-C chemokine receptor type 4 (CCR4) | Target Info | Antagonist | [1] |
| KEGG Pathway | Cytokine-cytokine receptor interaction | |||
| Chemokine signaling pathway | ||||
| Viral carcinogenesis | ||||
| Panther Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | |||
| Reactome | Chemokine receptors bind chemokines | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05399368) A Phase 2 Study to Evaluate the Efficacy and Safety of RPT193 as Monotherapy in Adults With Moderate-to-Severe Atopic Dermatitis. U.S.National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

