Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D5FSV8
|
|||
| Drug Name |
TRx0237
|
|||
| Synonyms |
951131-15-0; UNII-E79ZM68IOZ; E79ZM68IOZ; Leucomethylene Blue dihydrobromide; TRX0237 dihydrobromide; TRX 0237 dihydrobromide; TRX-0237 dihydrobromide; TRx0237(LMTX); TRX-0237 2HBr; Leukomethylene Blue dihydrobromide; Hydromethylthionine HBr(TRX0237); BCP24159; EX-A4299; Reduced methylene Blue dihydrobromide; N3,N3,N7,N7-Tetramethyl-10H-phenothiazine-3,7-diamine dihydrobromide; Leucomethylene Blue 2HBr;TRX0237 dihydrobromide;TRX 0237 dihydrobromide;TRX-0237 dihydrobromide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 3 | [1] | |
| Company |
TauRx Therapeutics
|
|||
| Structure |
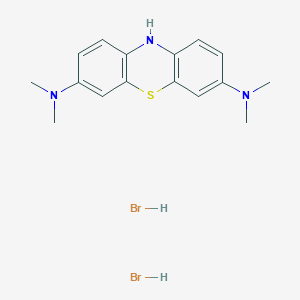 |
Download2D MOL
|
||
| Formula |
C16H21Br2N3S
|
|||
| Canonical SMILES |
CN(C)C1=CC2=C(C=C1)NC3=C(S2)C=C(C=C3)N(C)C.Br.Br
|
|||
| InChI |
1S/C16H19N3S.2BrH/c1-18(2)11-5-7-13-15(9-11)20-16-10-12(19(3)4)6-8-14(16)17-13;;/h5-10,17H,1-4H3;2*1H
|
|||
| InChIKey |
JAUPSVVTGFBHTN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 951131-15-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Microtubule-associated protein tau (MAPT) | Target Info | Inhibitor | [2] |
| KEGG Pathway | MAPK signaling pathway | |||
| Alzheimer's disease | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| EGFR1 Signaling Pathway | ||||
| Pathway Interaction Database | LPA receptor mediated events | |||
| Reelin signaling pathway | ||||
| Reactome | Caspase-mediated cleavage of cytoskeletal proteins | |||
| WikiPathways | Notch Signaling Pathway | |||
| IL-2 Signaling Pathway | ||||
| MAPK Signaling Pathway | ||||
| Copper homeostasis | ||||
| Kit receptor signaling pathway | ||||
| BDNF signaling pathway | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| Alzheimers Disease | ||||
| Regulation of Microtubule Cytoskeleton | ||||
| Apoptotic execution phase | ||||
| IL-5 Signaling Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01689246) Safety and Efficacy Study Evaluating TRx0237 in Subjects With Mild to Moderate Alzheimer's Disease. U.S. National Institutes of Health. | |||
| REF 2 | Mechanisms of Anticholinesterase Interference with Tau Aggregation Inhibitor Activity in a Tau-Transgenic Mouse Model. Curr Alzheimer Res. 2020;17(3):285-296. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

