Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D4M8RS
|
|||
| Drug Name |
TNP-2092
|
|||
| Synonyms |
UNII-W2P7EF7O6O; W2P7EF7O6O; CBR-2092; CHEMBL4594404; 3-((E)-((4-((1-((3R)-1-(3-Carboxy-1-cyclopropyl-7-fluoro-9-methyl-4-oxo-4H-quinolizin-8-yl)-3-pyrrolidinyl)cyclopropyl)methylamino)-1-piperidinyl)imino)methyl)rifamycin; Rifamycin, 3-((E)-((4-((1-((3R)-1-(3-carboxy-1-cyclopropyl-7-fluoro-9-methyl-4-oxo-4H-quinolizin-8-yl)-3-pyrrolidinyl)cyclopropyl)methylamino)-1-piperidinyl)imino)methyl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Skin and skin-structure infection [ICD-11: 1F28-1G0Z] | Phase 2 | [1] | |
| Joint infection [ICD-11: FA10] | Phase 1 | [2] | ||
| Company |
TenNor Therapeutics
|
|||
| Structure |
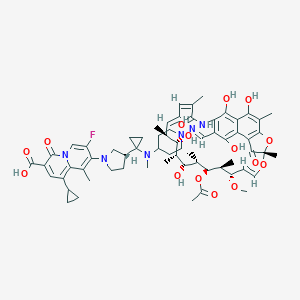 |
Download2D MOL
|
||
| Formula |
C65H81FN6O15
|
|||
| Canonical SMILES |
CC1C=CC=C(C(=O)NC2=C(C(=C3C(=C2O)C(=C(C4=C3C(=O)C(O4)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)C=NN5CCC(CC5)N(C)C6(CC6)C7CCN(C7)C8=C(C9=C(C=C(C(=O)N9C=C8F)C(=O)O)C1CC1)C)C
|
|||
| InChI |
1S/C65H81FN6O15/c1-31-13-12-14-32(2)61(80)68-50-44(56(77)47-48(57(50)78)55(76)37(7)59-49(47)60(79)64(9,87-59)85-26-20-46(84-11)33(3)58(86-38(8)73)36(6)54(75)35(5)53(31)74)28-67-71-24-18-41(19-25-71)69(10)65(21-22-65)40-17-23-70(29-40)52-34(4)51-42(39-15-16-39)27-43(63(82)83)62(81)72(51)30-45(52)66/h12-14,20,26-28,30-31,33,35-36,39-41,46,53-54,58,74-78H,15-19,21-25,29H2,1-11H3,(H,68,80)(H,82,83)/b13-12+,26-20+,32-14-,67-28+/t31-,33-,35+,36-,40+,46-,53-,54+,58+,64-/m0/s1
|
|||
| InChIKey |
OPZFMLLAJBIKAN-YSBZUPOXSA-N
|
|||
| CAS Number |
CAS 922717-97-3
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial DNA gyrase (Bact gyrase) | Target Info | Inhibitor | [3] |
| Bacterial DNA topoisomerase 4A (Bact parC) | Target Info | Inhibitor | [3] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03964493) TNP-2092 to Treat Acute Bacterial Skin and Skin Structure Infection (P2_ABSSSI). U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT04294862) Tissue Distribution, Pharmacokinetics, Safety, and Tolerability After a Single Dose of TNP-2092 in Participants Undergoing Primary Total Hip or Knee Arthroplasty. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of TenNor Therapeutics. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

