Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D3LZ2F
|
|||
| Drug Name |
Vemircopan
|
|||
| Synonyms |
Vemircopan; Vemircopan [INN]; 2086178-00-7; ACH-5228; ACH-0145228; HN6I4K50QB; 2-Azabicyclo(3.1.0)hexane-3-carboxamide, 2-(2-(3-acetyl-5-(2-methyl-5-pyrimidinyl)-1H-indazol-1-yl)acetyl)-N-(6-bromo-3-methyl-2-pyridinyl)-5-methyl-, (1R,3S,5R)-; 2-Azabicyclo[3.1.0]hexane-3-carboxamide, 2-[2-[3-acetyl-5-(2-methyl-5-pyrimidinyl)-1H-indazol-1-yl]acetyl]-N-(6-bromo-3-methyl-2-pyridinyl)-5-methyl-, (1R,3S,5R)-; VEMIRCOPAN [USAN]; UNII-HN6I4K50QB; CFD-IN-1; ALXN2050; CHEMBL4650325; SCHEMBL18578125; ALXN-2050; BDBM386545; GLXC-25930; US10287301, Compound 485; AKOS040757250; MS-30630; CS-0213583; ALXN2050; ACH 0145228; ACH-5228; (1R,3S,5R)-2-(2- (3-acetyl-5-(2- methylpyrimidin- 5-yl)-1H-indazol- 1-yl)acetyl)-N-(6- bromo-3- methylpyridin-2- yl)-5-methyl-2- azabicyclo[3.1.0] hexane-3- carboxamide; (1R,3S,5R)-2-(2-(3-Acetyl-5-(2-methylpyrimidin-5-yl)-1H-indazol-1-yl)acetyl)-N-(6-bromo-3-methylpyridin-2-yl)-5-methyl-2-azabicyclo(3.1.0)hexane-3-carboxamide; (1R,3S,5R)-2-[2-[3-acetyl-5-(2-methylpyrimidin-5-yl)indazol-1-yl]acetyl]-N-(6-bromo-3-methylpyridin-2-yl)-5-methyl-2-azabicyclo[3.1.0]hexane-3-carboxamide; (1R,3S,5R)-2-{[3-acetyl-5-(2-methylpyrimidin-5-yl)-1H-indazol-1-yl]acetyl}-N-(6-bromo-3-methylpyridin-2-yl)-5-methyl-2-azabicyclo[3.1.0]hexane-3-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Generalized myasthenia gravis [ICD-11: 8C60] | Phase 2 | [1] | |
| IgA nephropathy [ICD-11: MF8Y] | Phase 2 | [2] | ||
| Paroxysmal nocturnal haemoglobinuria [ICD-11: 3A21.0; ICD-10: D59.5] | Phase 2 | [3] | ||
| Proliferative lupus nephritis [ICD-11: 4A40] | Phase 2 | [2] | ||
| Company |
AstraZeneca
|
|||
| Structure |
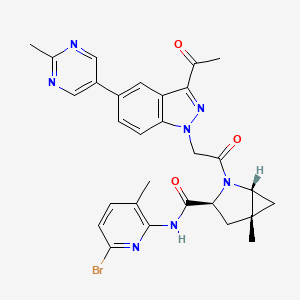 |
Download2D MOL |
||
| Formula |
C29H28BrN7O3
|
|||
| Canonical SMILES |
CC1=C(N=C(C=C1)Br)NC(=O)C2CC3(CC3N2C(=O)CN4C5=C(C=C(C=C5)C6=CN=C(N=C6)C)C(=N4)C(=O)C)C
|
|||
| InChI |
InChI=1S/C29H28BrN7O3/c1-15-5-8-24(30)33-27(15)34-28(40)22-10-29(4)11-23(29)37(22)25(39)14-36-21-7-6-18(19-12-31-17(3)32-13-19)9-20(21)26(35-36)16(2)38/h5-9,12-13,22-23H,10-11,14H2,1-4H3,(H,33,34,40)/t22-,23+,29-/m0/s1
|
|||
| InChIKey |
OCXAGXCMZACNEC-CTWZREHQSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Complement factor D (CFD) | Target Info | Inhibitor | [4] |
| Complement factor D (CFD) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Complement and coagulation cascades | |||
| Staphylococcus aureus infection | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Reactome | Platelet degranulation | |||
| Alternative complement activation | ||||
| WikiPathways | Complement and Coagulation Cascades | |||
| Human Complement System | ||||
| Adipogenesis | ||||
| Complement cascade | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05218096) A Phase 2, Randomized, Double-Blind, Placebo-Controlled Multicenter Study to Evaluate the Efficacy and Safety of ALXN2050 in Adult Participants With Generalized Myasthenia Gravis. U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT05097989) A Phase 2, Randomized, Double-blind, Placebo-controlled, Dose-finding Study to Evaluate the Efficacy and Safety of ALXN2050 in Adult Participants With Proliferative Lupus Nephritis (LN) or Immunoglobulin A Nephropathy (IgAN). U.S.National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT04170023) A Phase 2 Open-Label Proof of Concept Study to Assess the Efficacy, Safety, and Pharmacokinetics of the Oral Factor D (FD) Inhibitor ALXN2050 (ACH-0145228) in Paroxysmal Nocturnal Hemoglobinuria (PNH) Patients as Monotherapy. U.S.National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of AstraZeneca | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

