Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D1Z0IM
|
|||
| Drug Name |
IMR-687
|
|||
| Synonyms |
Tovinontrine; UNII-W248Y1AKOR; W248Y1AKOR; 2062661-53-2; a]pyrazin-8(7H)-one; CHEMBL4297290; SCHEMBL20358493; BDBM426313; US10513524, Compound (P3; 6-{(3S,4S)-4-methyl-1-[(pyrimidin-2-; yl)methyl]pyrrolidin-3-yl}-3-(oxan-4-yl)imidazo[1,5-; 6-((3S,4S)-4-Methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl)-3-tetrahydropyran-4-yl-7himidazo(1,5-a)pyrazin-8-one; Imidazo(1,5-a)pyrazin-8(7H)-one, 6-((3S,4S)-4-methyl-1-(2-pyrimidinylmethyl)-3-pyrrolidinyl)-3-(tetrahydro-2H-pyran-4-yl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Sickle-cell disorder [ICD-11: 3A51; ICD-10: D57, D57.8; ICD-9: 282.5, 282.6] | Phase 2 | [1] | |
| Company |
Imara
|
|||
| Structure |
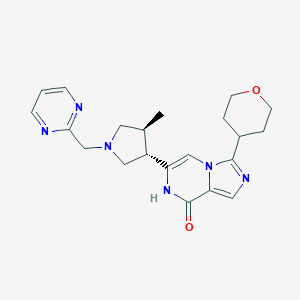 |
Download2D MOL |
||
| Formula |
C21H26N6O2
|
|||
| Canonical SMILES |
CC1CN(CC1C2=CN3C(=CN=C3C4CCOCC4)C(=O)N2)CC5=NC=CC=N5
|
|||
| InChI |
1S/C21H26N6O2/c1-14-10-26(13-19-22-5-2-6-23-19)11-16(14)17-12-27-18(21(28)25-17)9-24-20(27)15-3-7-29-8-4-15/h2,5-6,9,12,14-16H,3-4,7-8,10-11,13H2,1H3,(H,25,28)/t14-,16-/m1/s1
|
|||
| InChIKey |
GWGNPYYVGANHRJ-GDBMZVCRSA-N
|
|||
| CAS Number |
CAS 2062661-53-2
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 9 (PDE9) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Purine metabolism | |||
| Pathway Interaction Database | Regulation of Androgen receptor activity | |||
| Reactome | cGMP effects | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03401112) A Study of IMR-687 in Adult Patients With Sickle Cell Anaemia (Homozygous HbSS or Sickle-beta0 Thalassemia). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of Imara. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

