Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Z4JG
|
|||
| Former ID |
DNC010105
|
|||
| Drug Name |
4-methoxynaphthalen-1-amine
|
|||
| Synonyms |
4-methoxynaphthalen-1-amine; 16430-99-2; 1-Naphthalenamine, 4-methoxy-; 1-amino-4-methoxynaphthalene; CHEMBL572058; JVMUPDOMGALPOW-UHFFFAOYSA-N; F2146-0572; 4-methoxy naphthylamine; ACMC-209dqv; 4-methoxy-1-naphthalenamine; 1-methoxy-4-naphthalenamine; SCHEMBL331453; 1 -amino-4-methoxy-naphthalene; CTK8B0935; DTXSID80454284; ZINC11919966; BDBM50303938; ANW-22085; AKOS009236876; MCULE-9788991699; 4-methoxynaphthalen-1-amine, AldrichCPR; CJ-13724; AJ-60498; TC-110830; FT-0750226
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
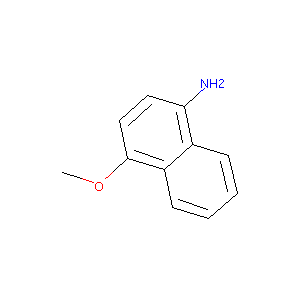 |
Download2D MOL |
||
| Formula |
C11H11NO
|
|||
| Canonical SMILES |
COC1=CC=C(C2=CC=CC=C21)N
|
|||
| InChI |
1S/C11H11NO/c1-13-11-7-6-10(12)8-4-2-3-5-9(8)11/h2-7H,12H2,1H3
|
|||
| InChIKey |
JVMUPDOMGALPOW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 16430-99-2
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Indoleamine 2,3-dioxygenase 1 (IDO1) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation | ||||
| L-kynurenine degradation | ||||
| Tryptophan degradation to 2-amino-3-carboxymuconate semialdehyde | ||||
| NAD de novo biosynthesis | ||||
| KEGG Pathway | Tryptophan metabolism | |||
| Metabolic pathways | ||||
| African trypanosomiasis | ||||
| NetPath Pathway | TSLP Signaling Pathway | |||
| IL5 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Tryptophan Metabolism | |||
| Reactome | Tryptophan catabolism | |||
| WikiPathways | Tryptophan metabolism | |||
| Metabolism of amino acids and derivatives | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem. 2010 Feb 11;53(3):1172-89. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

