Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Z2UQ
|
|||
| Former ID |
DNCL002158
|
|||
| Drug Name |
AZD2014
|
|||
| Synonyms |
AZD2014; 1009298-59-2; Vistusertib; AZD-2014; AZD 2014; UNII-0BSC3P4H5X; 0BSC3P4H5X; cc-551; 3-[2,4-Bis((3S)-3-methylmorpholin-4-yl)pyrido[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; CHEMBL2336325; 3-[2,4-Bis((3S)-3-methyLmorpholin-4-yl)pyrido-[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; C25H30N6O3; 3-(2,4-bis((S)-3-methylmorpholino)pyrido[2,3-d]pyrimidin-7-yl)-N-methylbenzamide; 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide; Vistusertib [INN]; Vistusertib [USAN]; Vistusertib (JAN/INN)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [1], [2] | |
| Company |
AstraZeneca
|
|||
| Structure |
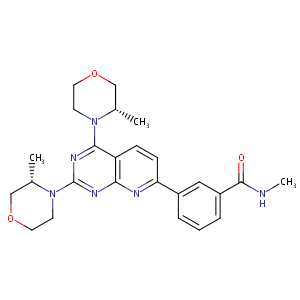 |
Download2D MOL |
||
| Formula |
C25H30N6O3
|
|||
| Canonical SMILES |
CC1COCCN1C2=NC(=NC3=C2C=CC(=N3)C4=CC(=CC=C4)C(=O)NC)N5CCOCC5C
|
|||
| InChI |
1S/C25H30N6O3/c1-16-14-33-11-9-30(16)23-20-7-8-21(18-5-4-6-19(13-18)24(32)26-3)27-22(20)28-25(29-23)31-10-12-34-15-17(31)2/h4-8,13,16-17H,9-12,14-15H2,1-3H3,(H,26,32)/t16-,17-/m0/s1
|
|||
| InChIKey |
JUSFANSTBFGBAF-IRXDYDNUSA-N
|
|||
| CAS Number |
CAS 1009298-59-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
58097037, 85072199, 137085461, 141539022, 144115665, 152159595, 152258658, 160647494, 162011892, 164045802, 164149381, 164158576, 172650682, 185990497, 198984517, 223366032, 223389028, 223702573, 223705079, 226633861, 241383711, 242060353, 248835190, 251962984, 252214932, 252450566, 252543438, 252552002
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7699). | |||
| REF 2 | ClinicalTrials.gov (NCT01793636) A Study Comparing AZD2014 vs Everolimus in Patients With Metastatic Renal Cancer. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Dramatic suppression of colorectal cancer cell growth by the dual mTORC1 and mTORC2 inhibitor AZD-2014. Biochem Biophys Res Commun. 2014 Jan 10;443(2):406-12. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

