Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Z1FX
|
|||
| Former ID |
DAP001019
|
|||
| Drug Name |
Estriol
|
|||
| Synonyms |
Aacifemine; Destriol; Estratriol; Estriel; Estriolo; Gynaesan; Hemostyptanon; Holin; Hormomed; Hormonin; Klimoral; Oestratriol; Oestriol; Oestriolum; Orestin; Orgastyptin; Overstin; Ovestin; Ovestrion; Stiptanon; Synapause; Theelol; Thulol; Tridestrin; Trihydroxyestrin; Trihydroxyoestrin; Triodurin; Triovex; Deuslon A; Estriolo [Italian]; Folicular hormone; Follicular hormone hydrate; Oestriol [Steroidal oestrogens]; A 13610; E0218; OE3; Deuslon-A; Estriel (TN); Estriol [USAN:JAN]; Estriol, unconjugated; Ortho-Gynest; Estriol (JP15/USP); Estra-1,3,5(10)-trien-3,16alpha,17beta-triol; Estra-1,3,5(10)-triene-3,16,17-triol; Estra-1,3,5(10)-triene-3,16alpha,17beta-triol; Oestra-1,3,5(10)-triene-3,16alpha,17beta-triol; Oestra-1,3,5(10)-triene-3,16-alpha,17-beta-triol; Estra-1,3,5(10)-trien-3,16.alpha., 17.beta.-triol; Estra-1,3,5(10)-trien-3,16.alpha.,17.beta.-triol; Estra-1,3,5(10)-triene-3,16.alpha., 17.beta.-triol; Estra-1,3,5(10)-triene-3,16.alpha.,17.beta.-triol; Oestra-1,3,5(10)-triene-3,16.alpha., 17.beta.-triol; Oestra-1,3,5(10)-triene-3,16.alpha.,17.beta.-triol; (16.alpha.,17.beta.)-Estra-1,3,5(10)-triene-3,16,17-triol; (16.alpha.,17.beta.)-Oestra-1,3,5(10)-triene-3,16,17-triol; (16alpha,17beta)-Estra-1,3,5(10)-triene-3,16,17-triol; (16alpha,17beta)-Oestra-1,3,5(10)-triene-3,16,17-triol; 1,3,5(10)-ESTRATRIENE-3,16,17-TRIOL; 1,3,5(10)-Estratriene-3,16-alpha,17beta-triol; 1,3,5(10)-Estratriene-3,16.alpha., 17.beta.-triol; 1,3,5(10)-Estratriene-3,16.alpha.,17.beta.-triol; 1,3,5(10)-Estratriene-3,16alpha,17beta-Triol; 1,3,5-Estratriene-3.beta.,16-.alpha.,17-.beta.-triol; 1,3,5-Estratriene-3beta,16alpha,17beta-triol; 1,3,5-Oestratriene-3-.beta.,16.alpha.,17.beta.-triol; 1,3,5-Oestratriene-3beta,16alpha,17beta-triol; 16,17-Epiestriol; 16-Epiestriol; 16-Hydroxyestradiol; 16-alpha,17-beta-Estriol; 16-alpha,17-beta-Oestriol; 16-alpha-Hydroxyestradiol; 16-alpha-Hydroxyoestradiol; 16.alpha.,17.beta.-Estriol; 16.alpha.,17.beta.-Oestriol; 16.alpha.-Estriol; 16.alpha.-Hydroxy-17.beta.-estradiol; 16.alpha.-Hydroxyestradiol; 16.alpha.-Hydroxyoestradiol; 16alpha,17beta-Estriol; 16alpha,17beta-Oestriol; 16alpha-Hydroxy-17beta-estradiol; 16alpha-Hydroxyestradiol; 16alpha-Hydroxyoestradiol; 3,16-alpha,17-beta-Estriol; 3,16-alpha,17-beta-Oestriol; 3,16-alpha,17-beta-Trihydroxy-delta-1,3,5-estratriene; 3,16-alpha,17-beta-Trihydroxy-delta-1,3,5-oestratriene; 3,16-alpha,17-beta-Trihydroxyestra-1,3,5(10)-triene; 3,16-alpha,17-beta-Trihydroxyoestra-1,3,5(10)-triene; 3,16.alpha.,17.beta.-Estriol; 3,16.alpha.,17.beta.-Trihydroxy-.delta.-1,3,5-estratriene; 3,16.alpha.,17.beta.-Trihydroxy-.delta.-1,3,5-oestratriene; 3,16.alpha.,17.beta.-Trihydroxy-1,3,5(10)-estratriene; 3,16.alpha.,17.beta.-Trihydroxyestra-1,3,5(10)-triene; 3,16alpha,17beta-Estriol; 3,16alpha,17beta-Trihydroxy-1,3,5(10)-estratriene; 3,16alpha,17beta-Trihydroxy-delta-1,3,5-oestratriene; 3,16alpha,17beta-trihydroxy-Delta(1,3,5)-estratriene
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hormone deficiency [ICD-11: 5A61.1; ICD-9: 340] | Approved | [1], [2], [3] | |
| Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Phase 2 | [4] | ||
| Therapeutic Class |
Estrogens
|
|||
| Company |
Pipex Pharmaceuticals
|
|||
| Structure |
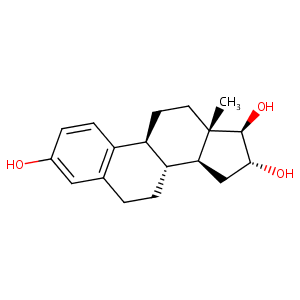 |
Download2D MOL |
||
| Formula |
C18H24O3
|
|||
| Canonical SMILES |
CC12CCC3C(C1CC(C2O)O)CCC4=C3C=CC(=C4)O
|
|||
| InChI |
1S/C18H24O3/c1-18-7-6-13-12-5-3-11(19)8-10(12)2-4-14(13)15(18)9-16(20)17(18)21/h3,5,8,13-17,19-21H,2,4,6-7,9H2,1H3/t13-,14-,15+,16-,17+,18+/m1/s1
|
|||
| InChIKey |
PROQIPRRNZUXQM-ZXXIGWHRSA-N
|
|||
| CAS Number |
CAS 50-27-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7560, 76927, 584764, 841394, 7847253, 7887358, 7979190, 8137982, 8153533, 11467124, 11468244, 11486792, 11532998, 11533161, 12146109, 14714952, 14775632, 24870296, 24894391, 24894396, 29224792, 46505881, 47213247, 47574682, 47796320, 48020302, 48170725, 48319784, 48421872, 49699292, 49965926, 50123986, 50280479, 53788422, 56313619, 57322932, 57392874, 57650833, 85788080, 87569501, 92126038, 92297526, 92309096, 93165576, 103469357, 103914539, 104253633, 104310166, 121363709, 124757812
|
|||
| ChEBI ID |
CHEBI:27974
|
|||
| ADReCS Drug ID | BADD_D00829 | |||
| SuperDrug ATC ID |
G03CA04; G03CC06
|
|||
| SuperDrug CAS ID |
cas=000050271
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2821). | |||
| REF 2 | Drug information of Estriol, 2008. eduDrugs. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026520) | |||
| REF 5 | Reprint of Are all estrogens the same Maturitas. 2008 Sep-Oct;61(1-2):195-201. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

