Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0YL8U
|
|||
| Former ID |
DNCL003321
|
|||
| Drug Name |
Omecamtiv mecarbil
|
|||
| Synonyms |
Omecamtiv mecarbil; 873697-71-3; Omecamtiv mecarbil (CK-1827452); CK-1827452; CK1827452; UNII-2M19539ERK; CK 1827452; methyl 4-(2-fluoro-3-(3-(6-Methylpyridin-3-yl)ureido)benzyl)piperazine-1-carboxylate; CHEMBL1800955; AMG-423; 2M19539ERK; Methyl 4-(2-Fluoro-3-{[(6-Methylpyridin-3-Yl)carbamoyl]amino}benzyl)piperazine-1-Carboxylate; Methyl 4-[[2-fluoro-3-[n'-(6-methylpyridin-3-yl)ureido]phenyl]methyl]piperazine-1-carboxylate; Omecamtiv mecarbil [USAN:INN]; omecamtiv mercarbil; MLS006011266; SCHEMBL400544; Omecamtiv Mecarbil;
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Heart failure [ICD-11: BD10-BD13; ICD-10: I50, I50.9; ICD-9: 428] | Phase 3 | [1] | |
| Company |
Amgen
|
|||
| Structure |
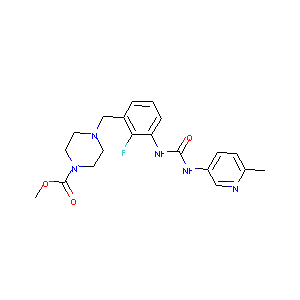 |
Download2D MOL |
||
| Formula |
C20H24FN5O3
|
|||
| Canonical SMILES |
CC1=NC=C(C=C1)NC(=O)NC2=CC=CC(=C2F)CN3CCN(CC3)C(=O)OC
|
|||
| InChI |
1S/C20H24FN5O3/c1-14-6-7-16(12-22-14)23-19(27)24-17-5-3-4-15(18(17)21)13-25-8-10-26(11-9-25)20(28)29-2/h3-7,12H,8-11,13H2,1-2H3,(H2,23,24,27)
|
|||
| InChIKey |
RFUBTTPMWSKEIW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 873697-71-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
16794251, 23784665, 42856803, 77786748, 99431601, 124360786, 124490388, 125164699, 134460814, 135263625, 136355109, 136367853, 136946631, 137415648, 144116311, 152258559, 160647394, 162038017, 162202760, 163835775, 164023440, 174007336, 185998834, 198937236, 223705270, 226729127, 242059893, 245117903, 251971205, 252088759, 252110190, 252160494, 252220161, 252451763
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cardiac myosin (MYBPC3) | Target Info | Modulator | [2] |
| KEGG Pathway | Hypertrophic cardiomyopathy (HCM) | |||
| Dilated cardiomyopathy | ||||
| Reactome | Striated Muscle Contraction | |||
| WikiPathways | Striated Muscle Contraction | |||
| Muscle contraction | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | CK-1827452, a sarcomere-directed cardiac myosin activator for acute and chronic heart disease. IDrugs. 2009 Apr;12(4):243-51. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

