Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0XT6W
|
|||
| Former ID |
DCL001117
|
|||
| Drug Name |
PDX-101
|
|||
| Synonyms |
Belinostat; 414864-00-9; PXD101; PXD-101; Belinostat (PXD101); Beleodaq; 866323-14-0; (E)-N-hydroxy-3-(3-(N-phenylsulfamoyl)phenyl)acrylamide; PXD 101; N-HYDROXY-3-(3-PHENYLSULFAMOYLPHENYL)ACRYLAMIDE; UNII-F4H96P17NZ; Belinostat(Random Configuration); NSC726630; PX105684; PX 105684; F4H96P17NZ; CHEBI:61076; (2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide; N-HYDROXY-3-[3-[(PHENYLAMINO)SULFONYL]PHENYL]-2-PROPENAMIDE; (E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide; PX-105684
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 2 | [1] | |
| Haematological malignancy [ICD-11: 2B33.Y] | Phase 1 | [2] | ||
| Peripheral T-cell lymphoma [ICD-11: 2A90.C; ICD-10: C84.4; ICD-9: 202.7] | Phase 1 | [2] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
National Cancer Institute (NCI)
|
|||
| Structure |
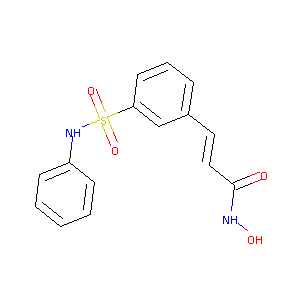 |
Download2D MOL |
||
| Formula |
C15H14N2O4S
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)NS(=O)(=O)C2=CC=CC(=C2)C=CC(=O)NO
|
|||
| InChI |
1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+
|
|||
| InChIKey |
NCNRHFGMJRPRSK-MDZDMXLPSA-N
|
|||
| CAS Number |
CAS 866323-14-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
12015640, 14899204, 17195013, 43530003, 48426953, 56374291, 57371991, 71821512, 79324295, 87226498, 87352001, 96025553, 99436973, 103572083, 104222411, 114788279, 124756987, 125163792, 126639499, 126671543, 126731246, 131480674, 134339486, 134964364, 135195315, 135727448, 136367285, 136368059, 136920289, 137008794, 142692247, 143496443, 144115689, 152234942, 152258138, 152344183, 160646977, 162011830, 162037424, 164041886, 164193975, 164202300, 170497642, 174529326, 175608161, 177748389, 179236375, 186022467, 187051798, 188899535
|
|||
| ChEBI ID |
CHEBI:61076
|
|||
| ADReCS Drug ID | BADD_D00225 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histone deacetylase (HDAC) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015 Mar;168(6):811-9. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

