Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0XS1W
|
|||
| Drug Name |
Lisinopril
|
|||
| Synonyms |
Acerbon; Acercomp; Alapril; Carace; Cipral; Cipril; Coric; Doneka; Hipril (TN); Inhibril; Inopril; LPR; Linopril; Linvas; Lipril; Lisinal; Lisinopril (INN); Lisinopril (anhydrous); Lisinopril anhydrous; Lisinoprilum; Lisinoprilum [Latin]; Lisipril; Lisoril; Lispril; Longes; Loril; Lysinopril; MK 521; MK 522; MK-521; Noperten; Novatec; Presiten; Prinil; Prinivil; Prinivil (TN); Sinopril; Sinopryl; Tensopril; Tensopril (TN); Tensyn; Tersif; Vivatec; Zestril; Zestril (TN)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Investigative | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Merck & Co
|
|||
| Structure |
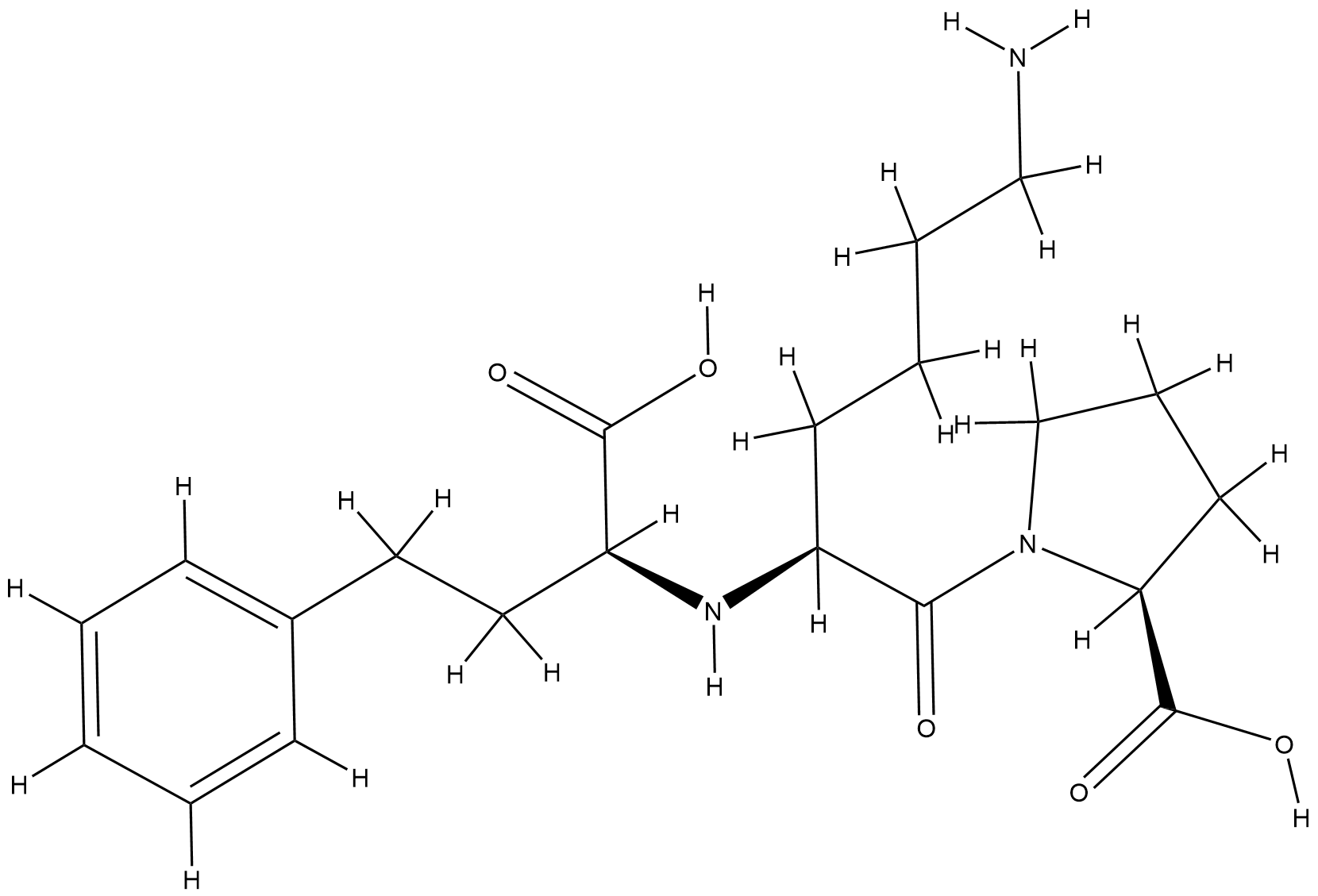 |
Download2D MOL |
||
| Formula |
C21H31N3O5
|
|||
| Canonical SMILES |
C1CC(N(C1)C(=O)C(CCCCN)NC(CCC2=CC=CC=C2)C(=O)O)C(=O)O
|
|||
| InChI |
1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1
|
|||
| InChIKey |
RLAWWYSOJDYHDC-BZSNNMDCSA-N
|
|||
| CAS Number |
CAS 76547-98-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7888691, 7979780, 8149783, 10322439, 11336212, 11361451, 11363391, 11365953, 11368515, 11372101, 11374737, 11376677, 11462423, 11466329, 11467449, 11485087, 11486217, 11489272, 11490756, 11492854, 11494311, 11654003, 12013163, 14806092, 14830773, 25622282, 26612244, 26680616, 26697064, 39384398, 46504893, 47515403, 47515404, 47810844, 47885506, 47885507, 48035230, 49698425, 49835323, 50019882, 50085996, 50124344, 53787084, 56311259, 57362085, 78195378, 81066263, 85787499, 87560323, 92124278
|
|||
| ChEBI ID |
CHEBI:43755
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN angiotensin-converting enzyme (ACE) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020 Apr 7;9(7):e016219. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

