Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0XP3W
|
|||
| Former ID |
DIB012223
|
|||
| Drug Name |
Ro-24-7429
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Phase 2 | [1] | |
| Company |
Roche Holding AG
|
|||
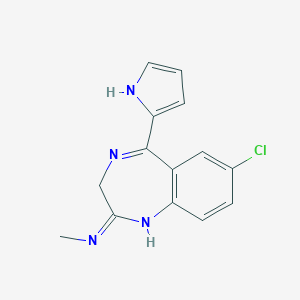
| Structure |
 |
Download2D MOL |
||
| Formula |
C14H13ClN4
|
|||
| Canonical SMILES |
CN=C1CN=C(C2=C(N1)C=CC(=C2)Cl)C3=CC=CN3
|
|||
| InChI |
1S/C14H13ClN4/c1-16-13-8-18-14(12-3-2-6-17-12)10-7-9(15)4-5-11(10)19-13/h2-7,17H,8H2,1H3,(H,16,19)
|
|||
| InChIKey |
LEAKQIXYSHIHCW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 139339-45-0
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:93522
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Tat protein (HIV tat) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00002314) A Study of Ro 24-7429 in Patients With HIV-Related Kaposi's Sarcoma. U.S. National Institutes of Health. | |||
| REF 2 | A randomized trial of the activity and safety of Ro 24-7429 (Tat antagonist) versus nucleoside for human immunodeficiency virus infection. The AIDS Clinical Trials Group 213 Team. J Infect Dis. 1995 Nov;172(5):1246-52. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

