Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0X8QO
|
|||
| Former ID |
DNCL001843
|
|||
| Drug Name |
PL-3994
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8] | Phase 2 | [1] | |
| Congestive heart failure [ICD-11: BD10; ICD-10: I50.0] | Phase 2 | [1] | ||
| Hypertension [ICD-11: BA00-BA04] | Phase 2 | [1] | ||
| Structure |
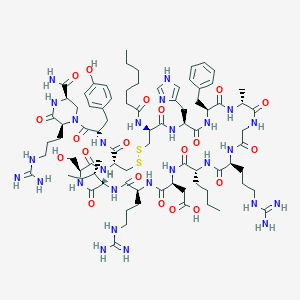 |
Download2D MOL
|
||
| Formula |
C82H127N27O20S2
|
|||
| Canonical SMILES |
CCCCCCC(=O)NC1CSSCC(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)CNC(=O)C(NC(=O)C(NC(=O)C(NC1=O)CC2=CNC=N2)CC3=CC=CC=C3)C)CCCNC(=N)N)CCCC)CC(=O)O)CCCNC(=N)N)C(C)CC)CO)C(=O)NC(CC4=CC=C(C=C4)O)C(=O)N5CC(NC(=O)C5CCCNC(=N)N)C(=O)N
|
|||
| InChI |
1S/C82H127N27O20S2/c1-6-9-11-15-25-62(112)98-59-41-130-131-42-60(76(126)104-56(34-47-26-28-49(111)29-27-47)79(129)109-39-57(66(83)116)105-77(127)61(109)24-18-32-93-82(88)89)107-74(124)58(40-110)106-78(128)65(44(4)8-3)108-70(120)52(23-17-31-92-81(86)87)100-73(123)55(36-64(114)115)103-69(119)51(21-10-7-2)99-68(118)50(22-16-30-91-80(84)85)97-63(113)38-94-67(117)45(5)96-71(121)53(33-46-19-13-12-14-20-46)101-72(122)54(102-75(59)125)35-48-37-90-43-95-48/h12-14,19-20,26-29,37,43-45,50-61,65,110-111H,6-11,15-18,21-25,30-36,38-42H2,1-5H3,(H2,83,116)(H,90,95)(H,94,117)(H,96,121)(H,97,113)(H,98,112)(H,99,118)(H,100,123)(H,101,122)(H,102,125)(H,103,119)(H,104,126)(H,105,127)(H,106,128)(H,107,124)(H,108,120)(H,114,115)(H4,84,85,91)(H4,86,87,92)(H4,88,89,93)/t44-,45+,50-,51+,52-,53-,54-,55-,56-,57+,58-,59+,60-,61-,65-/m0/s1
|
|||
| InChIKey |
HSHVZRILAMZYHY-WXSGKXQHSA-N
|
|||
| CAS Number |
CAS 952295-80-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Atrial natriuretic peptide receptor A (NPR1) | Target Info | Agonist | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00686803) Safety Study of Subcutaneously Administered PL-3994 in Subjects With Controlled Hypertension. U.S. National Institutes of Health. | |||
| REF 2 | In vitro and in vivo pharmacological profile of PL-3994, a novel cyclic peptide (Hept-cyclo(Cys-His-Phe-d-Ala-Gly-Arg-d-Nle-Asp-Arg-Ile-Ser-Cys)-Tyr-[Arg mimetic]-NH2) natriuretic peptide receptor-A agonist that is resistant to neutral endopeptidase and acts as a bronchodilator. Pulm Pharmacol Ther. 2013 April; 26(2): 229-238. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

