Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0X3GG
|
|||
| Former ID |
DNC001226
|
|||
| Drug Name |
Ro48-8071
|
|||
| Synonyms |
[4-({6-[Allyl(methyl)amino]hexyl}oxy)-2-fluorophenyl](4-bromophenyl)methanone; Ro 48-8071; 161582-11-2; Ro-48-8071; [4-({6-[allyl(methyl)amino]hexyl}oxy)-2-fluorophenyl](4-bromophenyl)methanone; CHEMBL304858; R048-8071; R71; 1gsz; (4-bromophenyl)[2-fluoro-4-({6-[methyl(prop-2-en-1-yl)amino]hexyl}oxy)phenyl]methanone; C23H27BRFNO2; {4-[6-(Allyl-methyl-amino)-hexyloxy]-2-fluoro-phenyl}-(4-bromo-phenyl)-methanone; (4-((6-(Allyl(methyl)amino)hexyl)oxy)-2-fluorophenyl)(4-bromophenyl)methanone; [4-[6-(allyl-methyl-amino)-hexyloxy]-2-fluoro-phenyl]-(4-bromo-phenyl)-methanone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
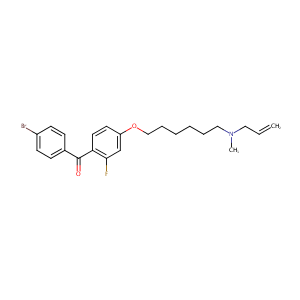 |
Download2D MOL |
||
| Formula |
C23H27BrFNO2
|
|||
| Canonical SMILES |
CN(CCCCCCOC1=CC(=C(C=C1)C(=O)C2=CC=C(C=C2)Br)F)CC=C
|
|||
| InChI |
1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3
|
|||
| InChIKey |
CMYCCJYVZIMDFU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 161582-11-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
584646, 801029, 5093993, 7890198, 8151349, 14784042, 29217636, 29221138, 46392034, 46505901, 76909598, 103066452, 103085819, 103258570, 104019858, 104299174, 117562407, 124893195, 125335089, 127341399, 127341400, 127648586, 135083264, 135246949, 135653432, 137255282, 140690287, 160965238, 162808377, 178103321, 179349273, 204360705, 225045161, 229670811, 252457668, 252480743, 252815121
|
|||
| ChEBI ID |
CHEBI:101064
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Lanosterol synthase (LSS) | Target Info | Inhibitor | [1], [2] |
| BioCyc | Cholesterol biosynthesis II (via 24,25-dihydrolanosterol) | |||
| Cholesterol biosynthesis III (via desmosterol) | ||||
| Cholesterol biosynthesis I | ||||
| Superpathway of cholesterol biosynthesis | ||||
| Lanosterol biosynthesis | ||||
| KEGG Pathway | Steroid biosynthesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| Panther Pathway | Cholesterol biosynthesis | |||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| Reactome | Cholesterol biosynthesis | |||
| Activation of gene expression by SREBF (SREBP) | ||||
| WikiPathways | Activation of Gene Expression by SREBP (SREBF) | |||
| SREBP signalling | ||||
| Cholesterol Biosynthesis | ||||
| Cholesterol biosynthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||
| REF 2 | Crystal structure of a squalene cyclase in complex with the potential anticholesteremic drug Ro48-8071. Chem Biol. 2002 May;9(5):639-45. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

