Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0WV3U
|
|||
| Former ID |
DAP000300
|
|||
| Drug Name |
Prazosin
|
|||
| Synonyms |
Furazosin; Justac; Lentopres; Prazocin; Prazosina; Prazosine; Prazosinum; Prazosin HCl; TNP00312; CP-12299; Hypovase (TN); Minipress (TN); Prazosin (INN); Prazosin [INN:BAN]; Prazosina [INN-Spanish]; Prazosine [INN-French]; Prazosinum [INN-Latin]; Vasoflex (TN); [3H]-Prazosin; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](furan-2-yl)methanone; [4-(4-amino-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-(2-furyl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone; Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-(9CI); Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)-(8CI); 1-(3-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanyl-carbonyl)piperazine hydrochloride; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine; 2-(4-(2-Furoyl)piperazin-1-yl)-4-amino-6,7-dimethoxyquinazoline; 2-[4-(2-furoyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine; 4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazinyl 2-furyl ketone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Benign prostatic hyperplasia [ICD-11: GA90; ICD-10: N40] | Approved | [1] | |
| Congestive heart failure [ICD-11: BD10; ICD-10: I50.0] | Approved | [1] | ||
| Hypertension [ICD-11: BA00-BA04] | Approved | [1] | ||
| Therapeutic Class |
Antihypertensive Agents
|
|||
| Company |
Pfizer Pharmaceuticals
|
|||
| Structure |
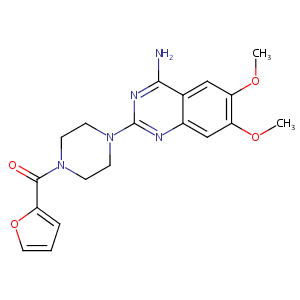 |
Download2D MOL |
||
| Formula |
C19H21N5O4
|
|||
| Canonical SMILES |
COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4=CC=CO4)N)OC
|
|||
| InChI |
1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22)
|
|||
| InChIKey |
IENZQIKPVFGBNW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 19216-56-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9572, 841974, 4500854, 7980376, 8153011, 11112649, 11112650, 11113367, 11120245, 11120733, 11121221, 11121703, 11122183, 11335550, 11360789, 11362790, 11363716, 11365352, 11366278, 11367914, 11368840, 11370831, 11370832, 11371821, 11373515, 11374104, 11376076, 11377002, 11406810, 11461761, 11466975, 11468095, 11485033, 11486809, 11489105, 11490367, 11492298, 11494636, 14804776, 26751613, 26751614, 29223971, 46508594, 47364941, 47364942, 47588782, 47662032, 47662033, 47662034, 47662035
|
|||
| ChEBI ID |
CHEBI:8364
|
|||
| ADReCS Drug ID | BADD_D01827 ; BADD_D01828 | |||
| SuperDrug ATC ID |
C02CA01
|
|||
| SuperDrug CAS ID |
cas=019216569
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor Alpha-1 (ADRA1) | Target Info | Antagonist | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Adventitia removal does not modify the alphaID-adrenoceptors response in aorta during hypertension and ageing. Auton Autacoid Pharmacol. 2009 Jul;29(3):117-33. | |||
| REF 3 | Characterization of alpha-adrenoceptor subtypes in smooth muscle of equine ileum. Am J Vet Res. 2001 Sep;62(9):1370-4. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

