Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0W5PN
|
|||
| Former ID |
DIB011530
|
|||
| Drug Name |
Radezolid
|
|||
| Synonyms |
Radezolid; Radezolid hydrochloride; RX-103; RX-1741; RX-01-667; Oxazolidinone antibiotic (bacterial infection), Rib-X Pharmaceuticals; Oxazolidinone antibiotic (CAP/uSSSI), Rib-X Pharamceuticals; Radezolid (iv, gram positive bacterial infection), Rib-X Pharmaceuticals; RX-1741 (iv, gram positive bacterial infection), Rib-X Pharmaceuticals; Radezolid (oral, CAP/uSSSI), Rib-X Pharmaceuticals; RX-1741 (oral, CAP/uSSSI), Rib-X Pharmaceuticals
Click to Show/Hide
|
|||
| Indication | Acne vulgaris [ICD-11: ED80; ICD-10: L70.0; ICD-9: 706.1] | Phase 2 | [1] | |
| Pneumonia [ICD-11: CA40] | Phase 2 | [2] | ||
| Company |
Melinta therapeutics
|
|||
| Structure |
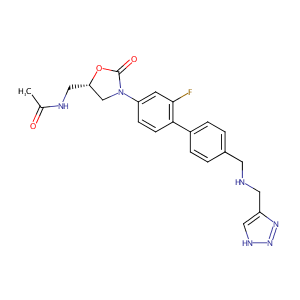 |
Download2D MOL |
||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial Protein synthesis (Bact PROS) | Target Info | Inhibitor | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | ClinicalTrials.gov (NCT00640926) Safety and Efficacy Study of Oxazolidinone to Treat Pneumonia. U.S. National Institutes of Health. | |||
| REF 3 | Antibiotics in the clinical pipeline in 2011. J Antibiot (Tokyo). 2011 Jun;64(6):413-25. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

