Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0W4HZ
|
|||
| Former ID |
DCL000152
|
|||
| Drug Name |
Lonafarnib
|
|||
| Synonyms |
Sarasar; Lonafarnib [USAN]; Sch 66336; Sch66336; SCH-066336; Sch-66336; Lonafarnib (USAN/INN); (+)-4-(2-(4-(11R)-3,10-Dibromo-8-chloro-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-piperidin-1-yl))-2-oxoethyl)-piperidine-1-carboxamide; (+)-4-(2-(4-(8-chloro-3,10-dibromo-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-1-piperidinyl)-2-oxoethyl)-1-piperidinecarboxamide; (+)-4[2-[4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo[5,6] cyclohepta[1,2-b]-pyridin-11(R)-yl-1-piperidinyl]-2-oxo-ethyl]-1-piperidinecarboxamide; 4-(2-(4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo(5,6)cyclohepta(1,2-b)pyridin-11-yl)-1-piperidinyl)-2-oxoethyl)-1-piperidinecarboxamide; 4-(2-(4-(8-Chloro-3,10-dibromo-6,11-dihydro-5H-benzo-(5,6)-cyclohepta(1,2-b)-pyridin-11(R)-yl)-1-piperidinyl)-2-oxo-ethyl)-1-piperidinecarboxamide; 4-{2-[4-(3,10-DIBROMO-8-CHLORO-6,11-DIHYDRO-5H-BENZO[5,6]CYCLOHEPTA[1,2-B]PYRIDIN-11-YL)PIPERIDIN-1-YL]-2-OXOETHYL}PIPERIDINE-1-CARBOXAMIDE
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hutchinson-Gilford progeria syndrome [ICD-11: LD2B] | Approved | [1] | |
| Myelodysplastic syndrome [ICD-11: 2A37; ICD-9: 238.7] | Phase 3 | [2] | ||
| Hepatitis D virus infection [ICD-11: 1E51.2; ICD-10: B16-B18] | Phase 2 | [3] | ||
| Non-small-cell lung cancer [ICD-11: 2C25.Y] | Discontinued in Phase 3 | [4], [5] | ||
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Schering-Plough
|
|||
| Structure |
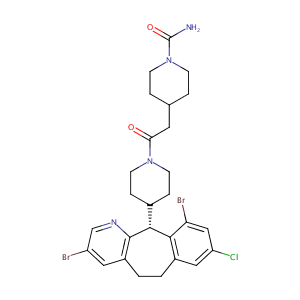 |
Download2D MOL |
||
| Formula |
C27H31Br2ClN4O2
|
|||
| Canonical SMILES |
C1CN(CCC1CC(=O)N2CCC(CC2)C3C4=C(CCC5=C3N=CC(=C5)Br)C=C(C=C4Br)Cl)C(=O)N
|
|||
| InChI |
1S/C27H31Br2ClN4O2/c28-20-12-19-2-1-18-13-21(30)14-22(29)24(18)25(26(19)32-15-20)17-5-9-33(10-6-17)23(35)11-16-3-7-34(8-4-16)27(31)36/h12-17,25H,1-11H2,(H2,31,36)/t25-/m1/s1
|
|||
| InChIKey |
DHMTURDWPRKSOA-RUZDIDTESA-N
|
|||
| CAS Number |
CAS 193275-84-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
535096, 828254, 7885229, 10249840, 12015147, 14839125, 14863416, 46225143, 46392748, 46514799, 47206583, 50067857, 53730874, 53790412, 57346725, 103241637, 104418463, 109692960, 126671481, 127339627, 127339628, 127339629, 127735655, 134338832, 134340513, 135105633, 135707678, 136895225, 137100866, 137263761, 142477936, 152159619, 152258621, 160647456, 162011930, 162205081, 162222422, 163884620, 164194111, 164761622, 170558848, 174007118, 174528153, 180372435, 186007024, 186014446, 198959604, 198993165, 223662989, 223704895
|
|||
| ChEBI ID |
CHEBI:47097
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Farnesyl protein transferase (Ftase) | Target Info | Modulator | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||
| REF 2 | ClinicalTrials.gov (NCT00109538) Study of Lonafarnib Versus Placebo in Subjects With Either Myelodysplastic Syndrome (MDS) or Chronic Myelomonocytic Leukemia (CMML) (Study P02978AM3)(TERMINATED). U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8024). | |||
| REF 5 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

