Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0W3ZN
|
|||
| Former ID |
DNC004311
|
|||
| Drug Name |
PD-169316
|
|||
| Synonyms |
PD 169316; 152121-53-4; PD-169316; PD169316; 4-(4-Fluorophenyl)-2-(4-nitrophenyl)-5-(4-pyridyl)-1H-imidazole; 4-(4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazol-5-yl)pyridine; 4-[4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazol-5-yl]pyridine; UNII-GX3Y2V80CV; PD168316; GX3Y2V80CV; CHEMBL17331; Pyridine, 4-(4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazol-5-yl)-; Pyridine, 4-[4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazol-5-yl]-; C20H13FN4O2; BGIYKDUASORTBB-UHFFFAOYSA-N; PubChem23987; AC1L1ISA
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
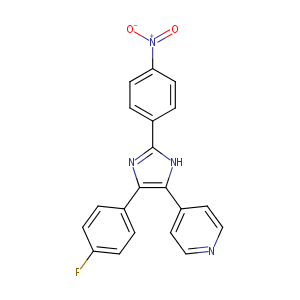 |
Download2D MOL |
||
| Formula |
C20H13FN4O2
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C2=NC(=C(N2)C3=CC=NC=C3)C4=CC=C(C=C4)F)[N+](=O)[O-]
|
|||
| InChI |
1S/C20H13FN4O2/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(8-4-15)25(26)27/h1-12H,(H,23,24)
|
|||
| InChIKey |
BGIYKDUASORTBB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 152121-53-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5426806, 8152893, 11120186, 11120674, 11121162, 11147269, 14876768, 15274767, 17404964, 24278156, 26753552, 26753553, 26759323, 29215153, 29223798, 48334228, 49655233, 53787724, 53800608, 56311900, 57322412, 72257528, 77337470, 85752747, 85788530, 90341746, 91722163, 99302800, 103181420, 104054749, 104307207, 121361128, 124891645, 125335119, 126671679, 126731456, 127511757, 134341516, 135327844, 135697755, 136048667, 136367835, 137046954, 142476820, 143499114, 144116351, 152133992, 152233483, 152344479, 162011905
|
|||
| ChEBI ID |
CHEBI:93358
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | LOX-5 messenger RNA (ALOX5 mRNA) | Target Info | Inhibitor | [2] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Arachidonic acid metabolism | |||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6016). | |||
| REF 2 | 2,4,5- triarylimidazole inhibitors of IL-1 biosynthesis, Bioorg. Med. Chem. Lett. 5(11):1171-1176 (1995). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

