Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0VK9O
|
|||
| Drug Name |
Sofpironium bromide
|
|||
| Synonyms |
BBI-4000; 1628106-94-4; Sofpironium bromide [USAN]; CHEMBL3707223; Sofpironium bromide (JAN/USAN/INN); D10989; Pyrrolidinium, 3-(((2R)-2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy)-1-(2-ethoxy-2-oxoethyl)-1-methyl-, bromide (1:1), (3R)-
Click to Show/Hide
|
|||
| Indication | Primary axillary hyperhidrosis [ICD-11: EE00.01] | Phase 3 | [1] | |
| Company |
Brickell Biotech Boulder, CO
|
|||
| Structure |
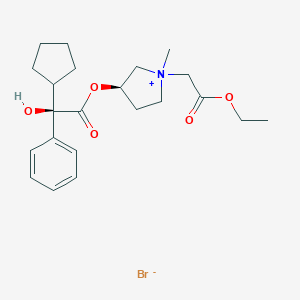 |
Download2D MOL |
||
| Formula |
C22H32BrNO5
|
|||
| Canonical SMILES |
CCOC(=O)C[N+]1(CCC(C1)OC(=O)C(C2CCCC2)(C3=CC=CC=C3)O)C.[Br-]
|
|||
| InChI |
1S/C22H32NO5.BrH/c1-3-27-20(24)16-23(2)14-13-19(15-23)28-21(25)22(26,18-11-7-8-12-18)17-9-5-4-6-10-17;/h4-6,9-10,18-19,26H,3,7-8,11-16H2,1-2H3;1H/q+1;/p-1/t19-,22+,23?;/m1./s1
|
|||
| InChIKey |
FIAFMTCUJCWADZ-JOFREBOKSA-M
|
|||
| CAS Number |
CAS 1628106-94-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cholinergic receptor unspecific (CHR) | Target Info | Antagonist | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03627468) A Safety Study of BBI-4000 Gel in Patients With Axillary Hyperhidrosis. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

