Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0VA0I
|
|||
| Former ID |
DCL000225
|
|||
| Drug Name |
Vorapaxar
|
|||
| Synonyms |
Vorapaxar; 618385-01-6; Zontivity; SCH-530348; SCH530348; UNII-ZCE93644N2; CHEMBL493982; CHEBI:82702; ZCE93644N2; MK-5348; ethyl ((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E)-2-(5-(3-fluorophenyl)pyridin-2-yl)vinyl)-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl)carbamate; Ethyl [(1r,3ar,4ar,6r,8ar,9s,9as)-9-{(E)-2-[5-(3-Fluorophenyl)pyridin-2-Yl]ethenyl}-1-Methyl-3-Oxododecahydronaphtho[2,3-C]furan-6-Yl]carbamate; Vorapaxar [USAN:INN]; Carbamic acid, [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)-2- pyridinyl]etheny
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Myocardial infarction [ICD-11: BA41-BA43; ICD-9: 410] | Approved | [1], [2], [3] | |
| Company |
Schering-Plough
|
|||
| Structure |
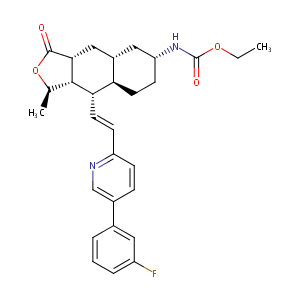 |
Download2D MOL |
||
| Formula |
C29H33FN2O4
|
|||
| Canonical SMILES |
CCOC(=O)NC1CCC2C(C1)CC3C(C2C=CC4=NC=C(C=C4)C5=CC(=CC=C5)F)C(OC3=O)C
|
|||
| InChI |
1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/b12-9+/t17-,20+,23-,24-,25+,26-,27+/m1/s1
|
|||
| InChIKey |
ZBGXUVOIWDMMJE-QHNZEKIYSA-N
|
|||
| CAS Number |
CAS 618385-01-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:82702
|
|||
| ADReCS Drug ID | BADD_D02368 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4047). | |||
| REF 2 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | In Process Citation. Pharm Unserer Zeit. 2009;38(4):320-8. | |||
| REF 5 | Novel antiplatelet strategies in acute coronary syndromes. Cleve Clin J Med. 2009 Apr;76 Suppl 1:S8-15. | |||
| REF 6 | Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet. 2009 Mar 14;373(9667):919-28. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

