Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V7WS
|
|||
| Former ID |
DNAP001402
|
|||
| Drug Name |
Zotarolimus
|
|||
| Synonyms |
Abt-578; Zotarolimus (TN)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Approved | [1], [2], [3] | |
| Company |
Medtronic
|
|||
| Structure |
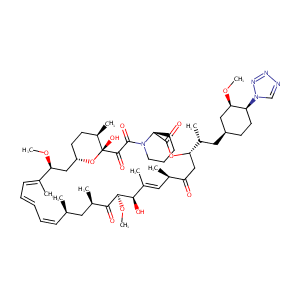 |
Download2D MOL
|
||
| Formula |
C52H79N5O12
|
|||
| Canonical SMILES |
CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)N5C=NN=N5)C)C)O)OC)C)C)C)OC
|
|||
| InChI |
1S/C52H79N5O12/c1-31-16-12-11-13-17-32(2)43(65-8)28-39-21-19-37(7)52(64,69-39)49(61)50(62)56-23-15-14-18-41(56)51(63)68-44(34(4)26-38-20-22-40(45(27-38)66-9)57-30-53-54-55-57)29-42(58)33(3)25-36(6)47(60)48(67-10)46(59)35(5)24-31/h11-13,16-17,25,30-31,33-35,37-41,43-45,47-48,60,64H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,32-17+,36-25+/t31-,33-,34-,35-,37-,38+,39+,40+,41+,43+,44+,45-,47-,48+,52-/m1/s1
|
|||
| InChIKey |
CGTADGCBEXYWNE-JUKNQOCSSA-N
|
|||
| CAS Number |
CAS 221877-54-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:135897
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7974). | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | ClinicalTrials.gov (NCT00589927) Triple Versus Dual Antiplatelet Therapy After ABT578-Eluting Stent. U.S. National Institutes of Health. | |||
| REF 4 | Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008 Jun;25(3):475-516. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

