Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0UL2L
|
|||
| Former ID |
DIB015995
|
|||
| Drug Name |
Lornoxicam
|
|||
| Synonyms |
CTX; Chlortenoxicam; Lorcam; Safem; Telos; Xefo; Xefo Rapid; HN-10000; TS-110; Ro-13-9297
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Approved | [1], [2] | |
| Company |
Roche Holding AG
|
|||
| Structure |
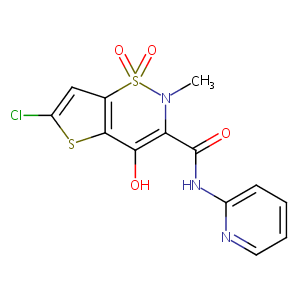 |
Download2D MOL |
||
| Formula |
C13H10ClN3O4S2
|
|||
| Canonical SMILES |
CN1C(=C(C2=C(S1(=O)=O)C=C(S2)Cl)O)C(=O)NC3=CC=CC=N3
|
|||
| InChI |
1S/C13H10ClN3O4S2/c1-17-10(13(19)16-9-4-2-3-5-15-9)11(18)12-7(23(17,20)21)6-8(14)22-12/h2-6,18H,1H3,(H,15,16,19)
|
|||
| InChIKey |
WLHQHAUOOXYABV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 70374-39-9
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:31783
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostaglandin G/H synthase (COX) | Target Info | Modulator | [1], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | ClinicalTrials.gov (NCT00997750) Efficacy and Safety of Lornoxicam in Patients With Acute Coronary Syndrome. U.S. National Institutes of Health. | |||
| REF 3 | The analgesic NSAID lornoxicam inhibits cyclooxygenase (COX)-1/-2, inducible nitric oxide synthase (iNOS), and the formation of interleukin (IL)-6 in vitro. Inflamm Res. 1999 Jul;48(7):369-79. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

