Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0U1OM
|
|||
| Former ID |
DAP000732
|
|||
| Drug Name |
Bromfenac
|
|||
| Synonyms |
Bromfenaco; Bromfenacum; Duract; Xibrom; BROMFENAC SODIUM; Bromfenac [INN]; Bromfenaco [Spanish]; Bromfenacum [Latin]; AHR-10282; Bromfenac (INN); Duract (TN); Xibrom (TN); Sodium 2-amino-3-(4-bromobenzoyl) phenylacetate sesquihydrate; [2-amino-3-(4-bromobenzoyl)phenyl]acetic acid; {2-amino-3-[(4-bromophenyl)carbonyl]phenyl}acetic acid; [2-Amino-3-(4-bromo-benzoyl)-phenyl]-acetic acid; 2-Amino-3-(4-bromobenzoyl)benzeneacetic acid; 2-[2-amino-3-(4-bromobenzoyl)phenyl]acetic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Postoperative inflammation [ICD-11: 1A00-CA43.1; ICD-10: T81; ICD-9: 998] | Approved | [1], [2] | |
| Inflammation [ICD-11: 1A00-CA43.1] | Withdrawn from market | [1], [3] | ||
| Therapeutic Class |
Antiinflammatory Agents
|
|||
| Structure |
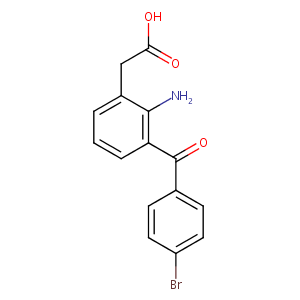 |
Download2D MOL |
||
| Formula |
C15H12BrNO3
|
|||
| Canonical SMILES |
C1=CC(=C(C(=C1)C(=O)C2=CC=C(C=C2)Br)N)CC(=O)O
|
|||
| InChI |
1S/C15H12BrNO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)
|
|||
| InChIKey |
ZBPLOVFIXSTCRZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 91714-94-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
8187024, 14802056, 43118081, 46508121, 50065379, 50714597, 51091863, 53786963, 57288753, 57314080, 85433311, 103293849, 104321485, 117562408, 119526091, 125536482, 129879365, 131330262, 134338019, 135065652, 137005805, 142970983, 152227509, 160814551, 160964302, 162197012, 163089933, 163373042, 163837609, 164117525, 164175212, 172092503, 175268177, 178103707, 179151433, 184545820, 186004941, 196396861, 203081294, 204420653, 223388494, 223435566, 223484710, 223551046, 223656326, 224730616, 226420876, 242065764, 249847324, 250181405
|
|||
| ChEBI ID |
CHEBI:240107
|
|||
| ADReCS Drug ID | BADD_D00300 ; BADD_D00301 | |||
| SuperDrug ATC ID |
S01BC11
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostaglandin G/H synthase 1 (COX-1) | Target Info | Inhibitor | [4] |
| BioCyc | C20 prostanoid biosynthesis | |||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Platelet activation | ||||
| Serotonergic synapse | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| Panther Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| Arachidonic acid metabolism | ||||
| Phase 1 - Functionalization of compounds | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7131). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021664. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin. 2006 Jun;22(6):1133-40. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

