Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T3CH
|
|||
| Former ID |
DNCL002576
|
|||
| Drug Name |
RGI-2001
|
|||
| Indication | Graft-versus-host disease [ICD-11: 4B24; ICD-9: 279.5] | Phase 2 | [1] | |
| Company |
REGiMMUNE
|
|||
| Structure |
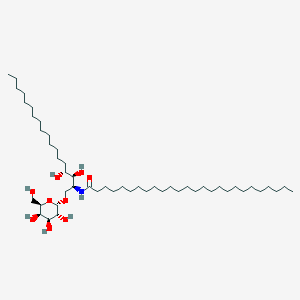 |
Download2D MOL
|
||
| Formula |
C50H99NO9
|
|||
| Canonical SMILES |
CCCCCCCCCCCCCCCCCCCCCCCCCC(=O)NC(COC1C(C(C(C(O1)CO)O)O)O)C(C(CCCCCCCCCCCCCC)O)O
|
|||
| InChI |
1S/C50H99NO9/c1-3-5-7-9-11-13-15-17-18-19-20-21-22-23-24-25-26-27-29-31-33-35-37-39-45(54)51-42(41-59-50-49(58)48(57)47(56)44(40-52)60-50)46(55)43(53)38-36-34-32-30-28-16-14-12-10-8-6-4-2/h42-44,46-50,52-53,55-58H,3-41H2,1-2H3,(H,51,54)/t42-,43+,44+,46-,47-,48-,49+,50-/m0/s1
|
|||
| InChIKey |
VQFKFAKEUMHBLV-BYSUZVQFSA-N
|
|||
| CAS Number |
CAS 158021-47-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:466659
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | T-cell receptor (TCR) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Pharmacologic expansion of donor-derived, naturally occurring CD4(+)Foxp3(+) regulatory T cells reduces acute graft-versus-host disease lethality without abrogating the graft-versus-leukemia effect in murine models. Biol Blood Marrow Transplant. 2011 Aug;17(8):1154-68. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

