Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T3AD
|
|||
| Former ID |
DAP000566
|
|||
| Drug Name |
Pentostatin
|
|||
| Synonyms |
Coforin; Covidarabine; Deoxycoformycin; Nipent; Oncopent; Vidarbine; Vira A deaminase inhibitor; CL-67310465; CO-Vidarabine; Co-V; Nipent (TN); PD-81565; PD-ADI; Pentostatin (JAN/USAN/INN); (8R)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol; (8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-7,8-dihydro-4H-imidazo[4,5-d][1,3]diazepin-8-ol; (R)-3-(2-Deoxy-.beta.-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo(4,5-d)(1,3)diazepin-8-ol; 2'-DCF; 2'-Deoxycoformycin; 2'-Dexoycoformycin
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hairy cell leukaemia [ICD-11: 2A82.2; ICD-10: C91.4; ICD-9: 202.4] | Approved | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Structure |
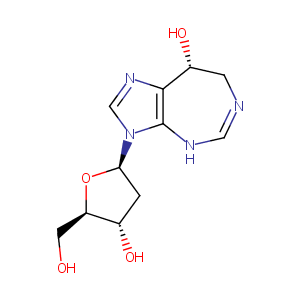 |
Download2D MOL |
||
| Formula |
C11H16N4O4
|
|||
| Canonical SMILES |
C1C(C(OC1N2C=NC3=C2NC=NCC3O)CO)O
|
|||
| InChI |
1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1
|
|||
| InChIKey |
FPVKHBSQESCIEP-JQCXWYLXSA-N
|
|||
| CAS Number |
CAS 53910-25-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5328, 627752, 7847223, 7886928, 7979039, 10298426, 12013121, 14823993, 14848291, 15221795, 15221797, 36883872, 46507116, 48416395, 49846739, 56459336, 57403140, 77345581, 93166294, 103589743, 104621158, 124893764, 127882252, 135003294, 135697915, 136342513, 137240504, 140705822, 141645553, 144206967, 152258942, 160647787, 160963897, 162173052, 163913892, 170498179, 174529719, 175267509, 178101507, 179116848, 184581996, 186021002, 223398588, 223769777, 226395166, 241375751, 249701474, 251916014, 251917362, 252156268
|
|||
| ADReCS Drug ID | BADD_D01730 | |||
| SuperDrug ATC ID |
L01XX08
|
|||
| SuperDrug CAS ID |
cas=053910251
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine deaminase (ADA) | Target Info | Inhibitor | [3], [4] |
| BioCyc | Purine nucleotides degradation | |||
| Purine deoxyribonucleosides degradation | ||||
| Purine ribonucleosides degradation to ribose-1-phosphate | ||||
| Adenosine nucleotides degradation | ||||
| Superpathway of purine nucleotide salvage | ||||
| Adenine and adenosine salvage III | ||||
| KEGG Pathway | Purine metabolism | |||
| Metabolic pathways | ||||
| Primary immunodeficiency | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| IL2 Signaling Pathway | ||||
| Panther Pathway | Adenine and hypoxanthine salvage pathway | |||
| Pathwhiz Pathway | Purine Metabolism | |||
| Pathway Interaction Database | p73 transcription factor network | |||
| C-MYB transcription factor network | ||||
| Validated transcriptional targets of deltaNp63 isoforms | ||||
| Validated transcriptional targets of TAp63 isoforms | ||||
| Reactome | Purine salvage | |||
| WikiPathways | Metabolism of nucleotides | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4805). | |||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 3 | Purine nucleoside analogs in indolent non-Hodgkin's lymphoma. Oncology (Williston Park). 2000 Jun;14(6 Suppl 2):13-5. | |||
| REF 4 | Acquisition of resistance to anticancer agents by overproduction of target enzymes. Nippon Rinsho. 1997 May;55(5):1030-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

