Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T1XW
|
|||
| Former ID |
DAP000795
|
|||
| Drug Name |
Meclizine
|
|||
| Synonyms |
Ancolan; Ancolon; Antivert; Bonadettes; Bonine; Calmonal; Chiclida; Diadril; Histamethine; Histamethizine; Histametizine; Histametizyn; Histametizyne; Itinerol; Marex; Meclicot; Meclozina; Meclozine; Meclozinum; Medivert; Monamine; Navicalm; Nevidoxine; Parachloramine; Peremesin; Postafene; Ravelon; Sabari; Siguran; Subari; Suprimal; Travelon; Veritab; Vomisseis; Vomissels; Dramamine II; Meclizine Hcl; UCB 170; UCB 5052; UCB 5062; Antivert (TN); Antivert/25; Antivert/50; Bonamine (TN); Bonine (TN); Meclizine [INN:BAN]; Meclozina [INN-Spanish]; Meclozine (BAN); Meclozinum [INN-Latin]; Neo-istafene; Neo-suprimal; Neo-suprimel; Nevidoxine (TN); Postafen (TN); Sea-Legs; Ru-Vert-M; U. C. B. 5062; U.C.B. 5062; (+-)-Meclizine; 1-((4-Chlorophenyl)phenylmethyl)-4-((3-methylphenyl)methyl)piperazine; 1-(p-Chloro-.alpha.-phenylbenzyl)-4-(m-methylbenzyl)piperazine; 1-(p-Chloro-alpha-phenylbenzyl)-4-(m-methylbenzyl)piperazine; 1-(p-Chlorobenzhydryl)-4-(m-methylbenzyl)diethylenediamine; 1-(p-Chlorobenzhydryl)-4-(m-methylbenzyl)piperazine; 1-[(4-chlorophenyl)(phenyl)methyl]-4-[(3-methylphenyl)methyl]piperazine; 1-[(4-chlorophenyl)-phenylmethyl]-4-[(3-methylphenyl)methyl]piperazine; 1-p-Chlorobenzhydryl-4-m-methylbenzylpiperazine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Dizziness [ICD-11: MB48; ICD-10: R11] | Approved | [1] | |
| Therapeutic Class |
Antiallergic Agents
|
|||
| Company |
Citron Pharma Llc
|
|||
| Structure |
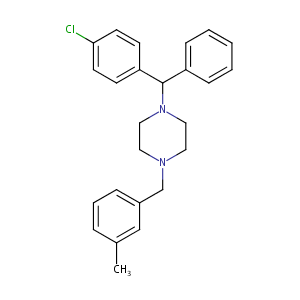 |
Download2D MOL |
||
| Formula |
C25H27ClN2
|
|||
| Canonical SMILES |
CC1=CC(=CC=C1)CN2CCN(CC2)C(C3=CC=CC=C3)C4=CC=C(C=C4)Cl
|
|||
| InChI |
1S/C25H27ClN2/c1-20-6-5-7-21(18-20)19-27-14-16-28(17-15-27)25(22-8-3-2-4-9-22)23-10-12-24(26)13-11-23/h2-13,18,25H,14-17,19H2,1H3
|
|||
| InChIKey |
OCJYIGYOJCODJL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 569-65-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9327, 442158, 4782133, 7979876, 8152525, 10527723, 11335582, 11360821, 11363785, 11366347, 11368909, 11371549, 11373612, 11377071, 11461793, 11466485, 11467605, 11483836, 11486209, 11487926, 11490301, 11491852, 11494705, 14805236, 29223145, 46507782, 47440184, 47515252, 47810681, 47959664, 48035039, 48110390, 48334421, 48416201, 49698867, 50012193, 50807781, 57322105, 76715647, 85209822, 85788825, 96024853, 103604960, 104305237, 117409993, 124883625, 124883627, 125312386, 125823630, 126689207
|
|||
| ChEBI ID |
CHEBI:6709
|
|||
| ADReCS Drug ID | BADD_D01361 ; BADD_D01362 | |||
| SuperDrug ATC ID |
R06AE05
|
|||
| SuperDrug CAS ID |
cas=000569653
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides vulgatus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides vulgatus was decreased by Meclozine dihydrochloride (adjusted p-values: 1.42E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Coriobacteriales | ||||
|
Studied Microbe: Collinsella aerofaciens
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Collinsella aerofaciens was decreased by Meclozine dihydrochloride (adjusted p-values: 3.51E-05). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Blautia obeum
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Blautia obeum was decreased by Meclozine dihydrochloride (adjusted p-values: 3.71E-04). | |||
|
Studied Microbe: Clostridioides difficile
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Clostridioides difficile was decreased by Meclozine dihydrochloride (adjusted p-values: 1.29E-03). | |||
|
Studied Microbe: Eubacterium eligens
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eubacterium eligens was decreased by Meclozine dihydrochloride (adjusted p-values: 1.30E-03). | |||
|
Studied Microbe: Eubacterium rectale
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eubacterium rectale was decreased by Meclozine dihydrochloride (adjusted p-values: 9.13E-03). | |||
|
Studied Microbe: Roseburia intestinalis
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Roseburia intestinalis was decreased by Meclozine dihydrochloride (adjusted p-values: 1.40E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Streptococcus salivarius
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Streptococcus salivarius was decreased by Meclozine dihydrochloride (adjusted p-values: 2.20E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Orphan nuclear receptor NR1I3 (NR1I3) | Target Info | Modulator | [3] |
| Pathway Interaction Database | Glucocorticoid receptor regulatory network | |||
| WikiPathways | Nuclear Receptors in Lipid Metabolism and Toxicity | |||
| Nuclear Receptors Meta-Pathway | ||||
| Constitutive Androstane Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 3 | Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR). Drug Metab Rev. 2006;38(1-2):51-73. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

